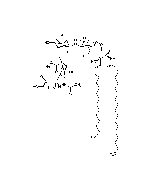Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
AdipoGen Life Sciences
Ganglioside GT1b . trisodium salt

| Product Details | |
|---|---|
| Synonyms | GT1b . 3Na; Trisialoganglioside GT1b . 3Na |
| Product Type | Chemical |
| Properties | |
| Formula |
C95H162N5O47 . 3Na |
| MW |
2126.3 . 69.0 (calculated on sphingosine C18:1 and stearic acid) |
| Sequence |
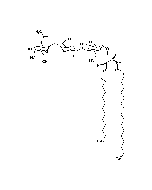
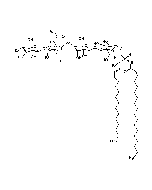
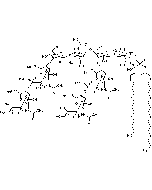
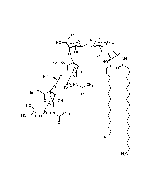
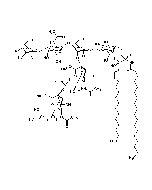
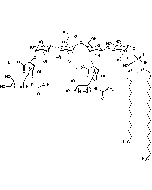
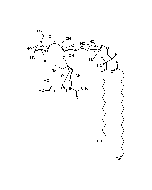
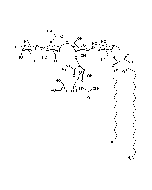
Structure: IV3Neu5AcII3NeuAc2GgOse4Cer; α-Neu5Ac-(2-3)-β-Gal-(1-3)-β-GalNAc-(1-4)-[α-Neu5Ac-(2-8)-α-Neu5Ac-(2-3)-]β-Gal-(1-4)-β-Glc-(1-1)-Cer; Cer: Sphingosine C18:1-C20:1, ~1:1 to 1:3 by vol.; stearic acid over 90% |
| CAS | 59247-13-1 |
| Source/Host Chemicals | Isolated from bovine brain. |
| Purity Chemicals | ≥98% (TLC) |
| Solubility | Soluble in water (micellar aggregates) or chloroform:methanol (2:1). |
| Formulation | Lyophilized |
| Endotoxin Content | Not detectable. |
| Identity | Determined by 1H-NMR, MS and HPTLC. |
| InChi Key | XWMACUIMZLECSZ-UVHDICHMSA-K |
| Smiles | [Na+].[Na+].[Na+].[H][C@@](O)(CO)[C@]([H])(O)C1O[C@@](CC(O)[C@H]1NC(C)=O)(O[C@H]1[C@H](O)C(CO)O[C@@H](OC2[C@@H](O)[C@H](CO)O[C@H](O[C@H]3C(CO)O[C@@H](O[C@H]4C(O)C(O)[C@H](OC[C@]([H])(NC(=O)CCCCCCCCCCCCCCCCC)[C@]([H])(O)\C=C\CCCCCCCCCCCCC)O[C@H]4CO)C(O)[C@H]3O[C@@]3(CC(O)[C@@H](NC(C)=O)C(O3)[C@@]([H])(O)[C@]([H])(CO)O[C@@]3(CC(O)[C@@H](NC(C)=O)C(O3)[C@@]([H])(O)[C@]([H])(O)CO)C([O-])=O)C([O-])=O)C2NC(C)=O)C1O)C([O-])=O |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice |
Hygroscopic. Protect from moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Gangliosides are acidic glycosphingolipids that form lipid rafts in the outer leaflet of the cell plasma membrane, especially in neuronal cells in the central nervous system. They participate in cellular proliferation, differentiation, adhesion, signal transduction, cell-to-cell interactions, tumorigenesis and metastasis. The accumulation of gangliosides has been linked to several diseases. Ganglioside GT1b binds botulinum and tetanus neurotoxins and it prevents neurotoxicity of glutamate on neuronal cells.
- Role of membrane gangliosides in the binding and action of bacterial toxins: P.H. Fishman; J. Membr. Biol. 69, 85 (1982).
- Binding of botulinum and tetanus neurotoxins to ganglioside GT1b and derivatives thereof: C.L. Schengrund, et al.; J. Neurochem. 57, 1024 (1991)
- Dynamic and structural properties of sphingolipids as driving force to the formation of membrane domains: S. Sonnino, et al.; Chem. Rev. 106, 2111 (2006)
- Involvement of ganglioside GT1b in glutamate release from neuroblastoma cells: S. Watanabe, et al.; Neurosci. Lett. 517, 140 (2012)
- The conserved arginine residue in all siglecs is essential for Siglec-7 binding to sialic acid: A. Yoshimura, et al.; BBRC in press, (2020)
- Analysis of biochemical features of ST8 α‑N‑acetyl‑neuraminide α2,8‑sialyltransferase (St8sia) 5 isoforms: E. Araki, et al.; Glycoconj. J. ahead of print (2022)