Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
BioViotica
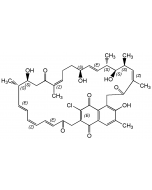
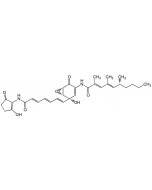
Manumycin B
115
CHF
CHF 115.00
In stock
BVT-0264-M0011 mgCHF 115.00

| Product Details | |
|---|---|
| Synonyms | UCF1A; N98-1272A |
| Product Type | Chemical |
| Properties | |
| Formula |
C28H34N2O7 |
| MW | 510.6 |
| CAS | 139023-58-8 |
| Source/Host Chemicals | Isolated from Streptomyces parvulus. |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | Yellow to brown powder. |
| Solubility | Soluble in DMSO, methanol or chloroform. |
| Identity | Determined by 1H-NMR and UV. |
| Declaration | Manufactured by BioViotica. |
| InChi Key | ZGICGDCGECBVTD-LOWJZYKPSA-N |
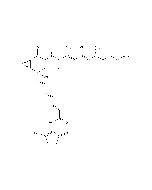
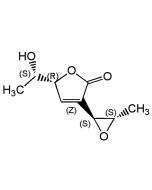
| Smiles | CCCC[C@@H](C)\C=C(/C)C(=O)NC1=C[C@@](O)(\C=C\C=C\C=C\C(=O)NC2=C(O)CCC2=O)[C@@H]2O[C@@H]2C1=O |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice |
After reconstitution protect from light at -20°C. Protect from light. |
| Use/Stability | Stable for at least 1 year after receipt when stored at -20°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
- Antibiotic.
- Antibacterial. Active against Gram-positive bacteria.
- Rasfarnesyltransferase inhibitor.
- Apoptosis (Caspase-1) inhibitor.
- AChE inhibitor.
- Anti-inflammatory.
Product References
- New compounds of the manumycin group of antibiotics and a facilitated route for their structure elucidation: I. Sattler, et al.; J. Org. Chem. 58, 6583 (1993)
- Identification of ras farnesyltransferase inhibitors by microbial screening: M. Hara, et al.; PNAS 90, 2281 (1993)
- TMC-1 A, B, C and D, new antibiotics of the Manumycin group produced by Streptomyces sp.: J. Kohno, et al.; J. Antibiot. 49, 1212 (1996)
- EI-1511-3, -5 and EI-1625-2, novel interleukin-1 beta converting enzyme inhibitors produced by Streptomyces sp. E-1511 and E-1625. III. Biochemical properties of EI-1511-3, -5 and EI-1625-2: T. Tanaka, et al.; J. Antibiot. 49, 1085 (1996)
- The manumycin-group metabolites: I. Sattler, et al.; Nat. Prod. Rep. 15, 221 (1998) (Review)
- The first total synthesis of a type II manumycin antibiotic, (+)-TMC-1 A: the total syntheses of (-)-LL-C10037β and(+)-manumycin B: J.J. Cronjé Grové, et al.; Chem. Commun. 1999, 421 (1999)
- Isolation and characterization of N98-1272 A, B and C, selective acetylcholinesterase inhibitors from metabolites ofan actinomycete strain: Z.-H. Zheng, et al.; J. Enzyme Inh. Med. Chem. 22, 43 (2007)
- Inhibition of Pro-Inflammatory Cytokines by Metabolites of Streptomycetes—A Potential Alternative to Current Anti-Inflammatory Drugs? J. Hrdy, et al.; Microorg. 8, 621 (2020)