Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
AdipoGen Life Sciences
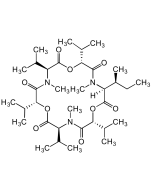
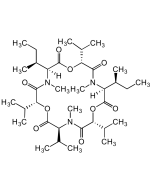
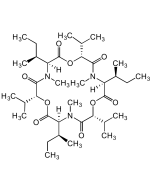
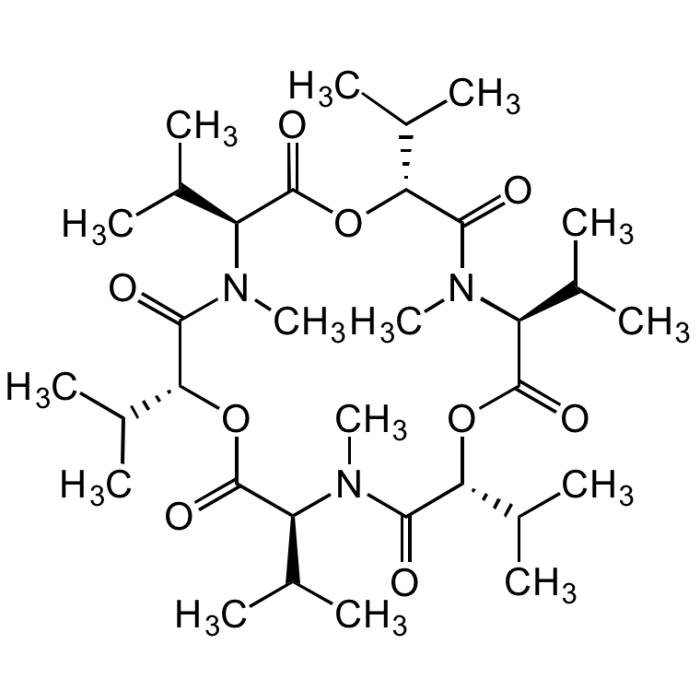
Enniatin B
As low as
170
CHF
CHF 170.00
In stock
Only %1 left
AG-CN2-0479-M0011 mgCHF 170.00
AG-CN2-0479-M0055 mgCHF 660.00
| Product Details | |
|---|---|
| Synonyms | Antibiotic 86/88 |
| Product Type | Chemical |
| Properties | |
| Formula |
C33H57N3O9 |
| MW | 639.8 |
| Merck Index | 14: 3585 |
| CAS | 917-13-5 |
| Source/Host Chemicals | Isolated from fungus Fusarium sp. |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | White to off-white solid. |
| Solubility | Soluble in DMSO, ethanol, methanol or DMF. Almost insoluble in water. |
| InChi Key | MIZMDSVSLSIMSC-VYLWARHZSA-N |
| Smiles | CC(C)[C@H]1OC(=O)[C@H](C(C)C)N(C)C(=O)[C@H](OC(=O)[C@H](C(C)C)N(C)C(=O)[C@H](OC(=O)[C@H](C(C)C)N(C)C1=O)C(C)C)C(C)C |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice |
Keep cool and dry. Protect from light. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
- Cyclohexadepsipeptide mycotoxin. One of four major analogs in the enniatin complex. Commonly found food contaminant in cereals and their products.
- Ionophore antibiotic (less ionophoric than Enniatin A). Incorporation into the cell membrane forms dimeric structures that transport monovalent ions across the membrane (especially the mitochondrial membranes) affecting oxidative phosphorylation uncoupling.
- Anticancer compound. Triggers apoptosis in several cancer cell lines.
- Moderate inhibitor of ACAT (acylcoenzyme A:cholesterolacyl transferase).
- Inhibitor of the pleiotropic drug resistance protein 5 (Pdr5p) in yeast.
- Shown to have a variety of other biological activities such as antifungal, anthelmintic, insecticidal, immunomodulatory and phytotoxic activity.
Product References
- Structure of the K+ complex with enniatin B, a macrocyclic antibiotic with K+ transport properties: M. Dobler, et al.; J. Mol. Biol. 42, 603 (1969)
- A comparison of beauvericin, enniatin and valinomycin as calcium transporting agents in liposomes and chromatophores: R.C. Prince, et al.; BBRC 59, 697 (1974)
- Inhibition of acyl-CoA: Cholesterol acyltransferase activity by cyclodepsipeptide antibiotics: H. Tomoda, et al.; J. Antibiot. (Tokyo) 45, 1626 (1992)
- Investigation of the electrophysiological properties of enniatins: M. Kamyar, et al.; Arch. Biochem. Biophys. 429, 215 (2004)
- Enniatin has a new function as an inhibitor of Pdr5p, one of the ABC transporters in Saccharomyces cerevisiae: K. Hiraga, et al.; BBRC 328, 1119 (2005)
- Cytotoxicity of enniatins A, A1, B, B1, B2 and B3 from Fusarium avenaceum: L. Ivanova, et al.; Toxicon 47, 868 (2006)
- Enniatin exerts p53-dependent cytostatic and p53-independent cytotoxic activities against human cancer cells: R. Dornetshuber, et al.; Chem. Res. Toxicol. 20, 465 (2007)
- Emerging Fusarium-mycotoxins fusaproliferin, beauvericin, enniatins, and moniliformin - a review: M. Jestoi; Crit. Rev. Food Sci. Nutr. 48, 21 (2008)
- Oxidative stress and DNA interactions are not involved in Enniatin- and Beauvericin-mediated apoptosis induction: R. Dornetshuber, et al.; Mol. Nutr. Food Res. 53, 1112 (2009)
- Enniatins A1, B and B1 from an endophytic strain of Fusarium tricinctum induce apoptotic cell death in H4IIE hepatoma cells accompanied by inhibition of ERK phosphorylation: W. Waetjen, et al.; Mol. Nutr. Food Res. 53, 431 (2009)
- Toxigenicity of enniatins from Western Australian Fusarium species to brine shrimp (Artemia franciscana): D.C. Tan, et al.; Toxicon 57, 817 (2011)
- Revisiting the enniatins: a review of their isolation, biosynthesis, structure determination and biological activities: A.A. Sy-Cordero, et al.; J. Antibiot. 65, 541 (2012)
- Quantitative determination of the Fusarium mycotoxins beauvericin, enniatin A, A1, B and B1 in pig plasma using high performance liquid chromatography-tandem mass spectrometry: M. Devreese, et al.; Talanta 106, 212 (2013)
- Enniatin A1, enniatin B1 and beauvericin on HepG2: Evaluation of toxic effects: A. Juan-Garcia, et al.; Food Chem. Toxicol. 84, 188 (2015)
- Beauvericin (BEA) and enniatin B (ENNB)-induced impairment of mitochondria and lysosomes - Potential sources of intracellular reactive iron triggering ferroptosis in Atlantic salmon primary hepatocytes: S. Söderström, et al.; Food Chem. Toxicol. 161, 112819 (2022)
- Dietary beauvericin and enniatin B exposure cause different adverse health effects in farmed Atlantic salmon: M.H.G. Berntssen, et al.; Food Chem. Toxicol. 113648 (2023)