Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
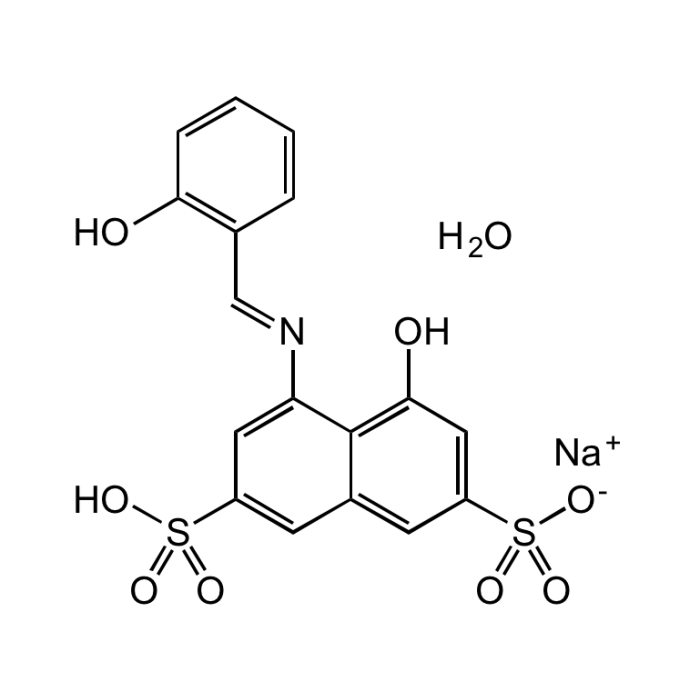
Azomethine-H monosodium salt hydrate
| Product Details | |
|---|---|
| Synonyms | 4-Hydroxy-5-(2-hydroxybenzylideneamino)-naphthalene-2,7-disulfonic acid monosodium salt hydrate; 4-Hydroxy-5-(salicylideneamino)-2,7-naphthalenedisulfonic acid monosodium salt |
| Product Type | Chemical |
| Properties | |
| Formula | C17H12NNaO8S2 . xH2O |
| MW | 445.40 (anhydrous basis) |
| CAS | 206752-32-1 |
| Source/Host Chemicals | Synthetic |
| Purity Chemicals | ≥95% (T) |
| Appearance | Yellow to orange powder. |
| Solubility | Soluble in water, ethanol or acetone. |
| Identity | Determined by NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | AAIGDXDVSZJVSW-IFJQNBRBSA-M |
| Smiles | O.[Na+].OC1=C(\C=N\C2=CC(=CC3=CC(=CC(O)=C23)S([O-])(=O)=O)S(O)(=O)=O)C=CC=C1 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | +4°C |
| Handling Advice |
Keep cool and dry. Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at +4°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Probe for the colorimetric determination of boron in samples such us soils, plants, composts, manure, water, nutirent solution, glass or steel (microgram levels of boron). It forms an orange complex with boron in aqueous solution (absorption maxima at ~415nm). The detection range of boron in sample solutions is 1.0-6ppm. To detect boron in plant samples EDTA is used to mask copper, iron and aluminium ions. It is also used in electrocyclization reactions in the synthesis of martinellic acid, spirotryprostatin A and benzodiazepinones. Was shown to produce free radicals and might have anti-malarial and anti-cancer properties.
(1) W.D. Basson, et al.; Analyst 94, 1135 (1969) | (2) R.R. Spencer & D.E. Erdmann; Environ. Sci. Technol. 13, 954 (1979) | (3) R.A. Edwards; Analyst 105, 139 (1980) | (4) M. Zenki, et al.; Fresenius J. Anal. Chem. 334, 238 (1989) | (5) J. Ciba & A. Chrusciel; Fresenius J. Anal. Chem. 342, 147 (1992) | (6) E.L. Novelli, et al.; Free Radic. Res. 26, 319 (1997) | (7) A. Gross, et al.; Chemosphere 72, 400 (2008) | (8) E. Merdivan, et al.; Spectrochim. Acta A Mol. Biomol. Spectrosc. 71, 2045 (2009)





