Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
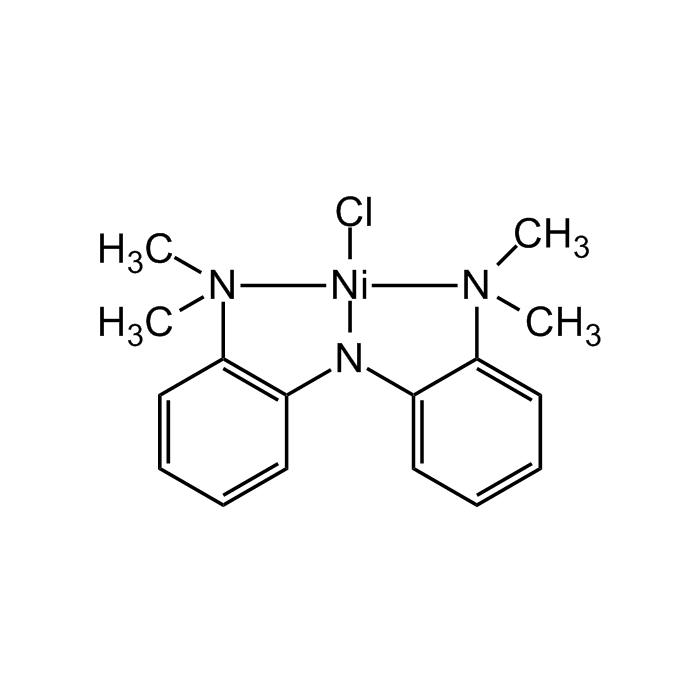
Nickamine
| Product Details | |
|---|---|
| Synonyms | Bis[(2-dimethylamino)phenyl]amine nickel(II) chloride; Chloro[N2-[2-(dimethylamino-κN)phenyl]-N1,N1-dimethyl-1,2-benzenediaminato-κN1,κN2]-nickel |
| Product Type | Chemical |
| Properties | |
| Formula |
C16H20ClN3Ni |
| MW | 348.5 |
| CAS | 1033772-47-2 |
| Source/Host Chemicals | Synthetic |
| Purity Chemicals | ≥95% (NMR) |
| Appearance | Grey powder. |
| Solubility | Soluble in DMSO, THF, 1,4-dioxane, chloroform, dichloromethane, DMA and toluene. Insoluble in alkanes and water. |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | IRLHVNUEEKJBEL-UHFFFAOYSA-M |
| Smiles | C[N]1(C)C2=CC=CC=C2N2C3=C(C=CC=C3)[N](C)(C)[Ni]12Cl |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | +4°C |
| Handling Advice |
Keep cool and dry. Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at +4°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Reagent used as a catalyst for cross-coupling reactions between alkyl halides and carbon nucleophiles. Catalyst for alkyl-alkyl Kumada coupling of secondary alkyl halides, direct alkylation of heterocyclic C-H bonds, Sonogashira coupling of nonactivated alkyl halides, Kumada-Corriu-Tamao coupling of nonactivated alkyl halides with aryl and heteroaryl nucleophiles. Reactant of pincer NN2 ligand leading to selective carbon-carbon bond formation.
(1) Z. Csok, et al.; JACS 130, 8156 (2008) | (2) O. Vechorkin, et al.; JACS 131, 9756 (2009) | (3) O. Vechorkin, et al.; JACS 131, 12078 (2009) | (4) O. Vechorkin & X. Hu; Angew. Chem. Inter. Ed. 48, 2937 (2009) | (5) O. Vechorkin, et al.; Angew. Chem. Inter. Ed. 49, 3061 (2010) | (6) P. Ren, et al.; JACS 133, 7084 (2011) | (7) X. Hu; Chimia 66, 154 (2012) | (8) J. Breitenfeld & X. Hu; Chimia 68, 235 (2014)