Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
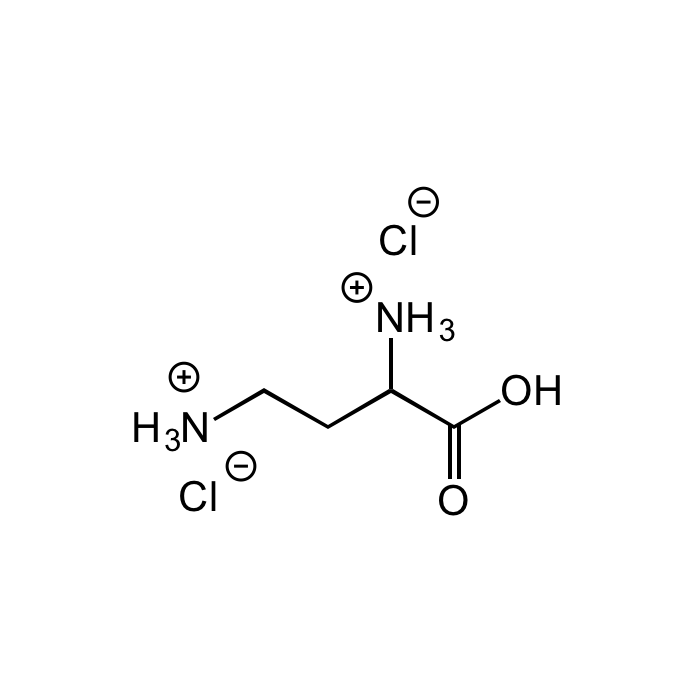
L-2,4-Diaminobutyric acid dihydrochloride
| Product Details | |
|---|---|
| Synonyms | (2S)-2,4-Diamino-butanoic acid dihydrochloride; H-Dab-OH |
| Product Type | Chemical |
| Properties | |
| Formula |
C4H10N2O2 . 2HCl |
| MW | 118.1 . 72.9 |
| CAS | 1883-09-6 |
| Source/Host Chemicals | Synthetic |
| Purity Chemicals | ≥95% (NMR) |
| Appearance | Yellowish powder. |
| Solubility | Soluble in water. |
| Identity | Determined by NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | CKAAWCHIBBNLOJ-UHFFFAOYSA-N |
| Smiles | [Cl-].[Cl-].[NH3+]CCC([NH3+])C(O)=O |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | +4°C |
| Handling Advice |
Keep cool and dry. Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at +4°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Unnatural amino acid derivative. Pharmacological tool and potential chiral building block used as an internal standard for amino acid analysis. Suitable reagent used for the differentiation of β-N-methylamino-L-alanine from the diamino acids by using HPLC-FD, UHPLC-UV, UHPLC-MS, and triple quadrupole tandem mass spectrometry (UHPLC-MS/MS). Used in the quantification of neurotoxin β-N-methylamino-L-alanine (BMAA) in seafood. It has been found to inhibit GABA transaminase (ABAT), producing elevated levels of GABA and to have an antitumor activity.
(1) J.F. Riordan & R.W. Giese; Meth. Enzymol. 47, 31 (1977) | (2) P.M. Beart, et al.; Neurosci. Lett. 5, 193 (1977) | (3) G. Ronquist, et al.; J. Cancer Res. Clin. Oncol. 96, 259 (1980) | (4) P.J. Blind, et al.; Anticancer Res. 23, 1245 (2003) | (5) M.G. Thomas; J. Bacteriol. 191, 4594 (2009) | (6) S.A. Banack, et al.; Toxicon. 57, 730 (2011) | (7) L. Jiang, et al.; Sci. Rep. 4, 6931 (2014)