Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
Difluoromethyl phenyl sulfone
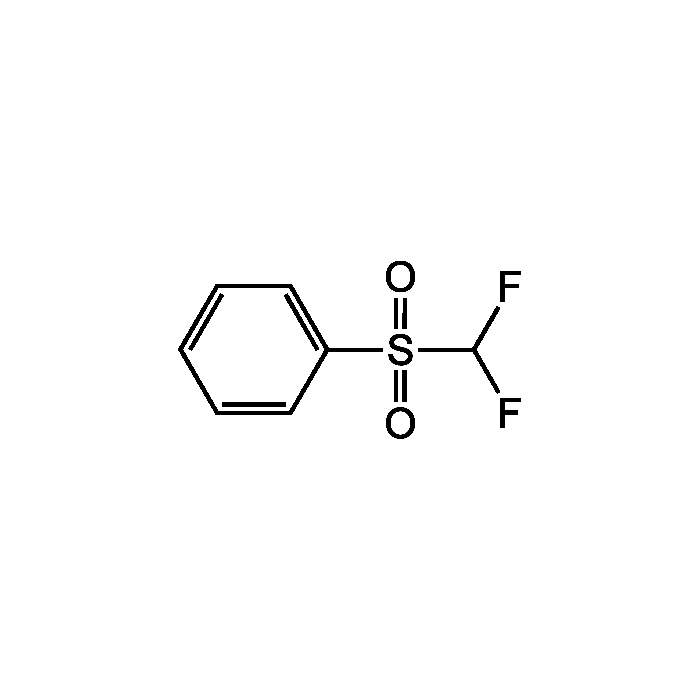
| Product Details | |
|---|---|
| Synonyms | Phenyl difluoromethyl sulfone |
| Product Type | Chemical |
| Properties | |
| Formula | C7H6F2O2S |
| MW | 192.2 |
| CAS | 1535-65-5 |
| Source/Host Chemicals | Synthetic. |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | Colorless to light yellow semi-solid or solid. |
| Solubility | Soluble in chloroform. |
| Identity | Determined by NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | LRHDNAVPELLXDL-UHFFFAOYSA-N |
| Smiles | FC(F)S(=O)(=O)C1=CC=CC=C1 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice |
Keep cool and dry. Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Building block for synthesis. Has been used for nucleophilic difluoro(phenylsulfonyl)methylation of carbonyls, reductive silylation and the preparation of trifluoro- and difluoromethylsilanes, fluoroalkylation/chloroalkylation of α,β-enones, arynes, acetylenic ketones and other Michael acceptors and difluoromethylation of primary alkyl halides . It is also used in preparation of α-difluoromethyl amines, anti-difluoropropanediols, β-difluoromethylated and β-difluoromethylenated alcohols and amines, difluoroalkenes, difluoromethyl alcohol derivatives and fluoromethylated vicinal ethylenediamines.
(1) G.K.S. Prakash, et al.; J. Org. Chem. 68, 4457 (2003) | (2) G.K.S. Prakash, et al.; Angew. Chem. 42, 5216 (2003) | (3) G.K.S. Prakash, et al.; Angew. Chem. 43, 5203 (2004) | (4) G.K.S. Prakash, et al.; Org. Lett. 6, 4315 (2004) | (5) Y. Li & J. Hu; Angew. Chem. 44, 5882 (2005) | (6) G.K.S. Prakash, et al.; Europ. J. Org. Chem. 11, 2218 (2005) | (7) C. Ni, et al.; Angew. Chem. 46, 786 (2007) | (8) J. Liu, et al.; J. Org. Chem. 72, 3119 (2007) | (9) C. Ni, et al.; J. Org. Chem. 73, 5699 (2008) | (10) M. Hu, et al.; J. Fluor. Chem. 155, 52 (2013)