Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
PTACH
| Product Details | |
|---|---|
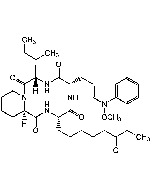
| Synonyms | NCH 51; Cpd 51; S-[6-(4-Phenyl-2-thiazolylcarbamoyl)hexyl] thioisobutyrate |
| Product Type | Chemical |
| Properties | |
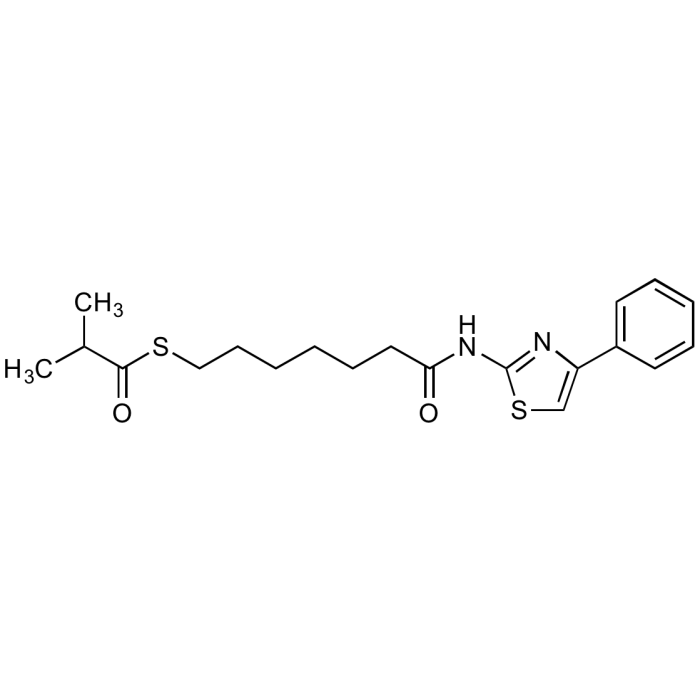
| Formula | C20H26N2O2S2 |
| MW | 390.56 |
| CAS | 848354-66-5 |
| Source/Host Chemicals | Synthetic. |
| Purity Chemicals | ≥95% (HPLC) |
| Appearance | White crystals. |
| Solubility | Soluble in DMSO (25mg/ml). |
| Identity | Determined by NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | MDYDGUOQFUQOGE-UHFFFAOYSA-N |
| Smiles | CC(C)C(=O)SCCCCCCC(=O)NC1=NC(=CS1)C1=CC=CC=C1 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | +4°C |
| Handling Advice |
Keep cool and dry. Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at +4°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Non-hydroxamate HDAC inhibitor (HDACi) (IC50: 32, 48 and 41nM for HDAC4, HDAC1 and HDAC6, respectively). Cell-permeable prodrug that is intracellularly converted to a potent HDAC inhibitor. Predicted to exhibit a similar HDAC binding mode as that of SAHA, interacting with the active-site zinc targeting group. Shown to exhibit comparable antiproliferative and apoptotic activity as SAHA against various cancer cell lines. Inhibits growth of various cancer cells in vitro (EC50 = 1.1 - 9.1μM). Also reactivates latent HIV-1 gene expression.
(1) T. Suzuki, et al.; J. Med. Chem. 48, 1019 (2005) | (2) T. Sanda, et al.; Leukemia 21, 2344 (2007) | (3) T. Suzuki, et al.; Bioorg. Med. Chem. Lett. 17, 1558 (2007) | (4) A.F. Victoriano, et al.; FEBS Lett. 585, 1103 (2011)