Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
BioViotica
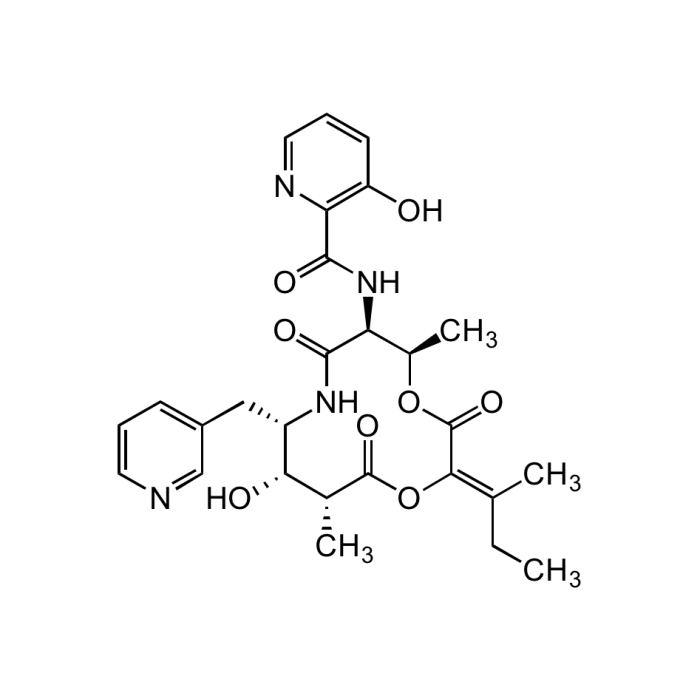
Pyridomycin
As low as
325
CHF
CHF 325.00
In stock
Only %1 left
BVT-0455-M0011 mgCHF 325.00
| Product Details | |
|---|---|
| Synonyms | Antibiotic U 24544; Erizomycin; NSC 246134 |
| Product Type | Chemical |
| Properties | |
| Formula |
C27H32N4O8 |
| MW | 540.6 |
| CAS | 18791-21-4 |
| Source/Host Chemicals | Isolated from Streptomyces sp. |
| Purity Chemicals | ≥98% (1H-NMR, HPLC) |
| Appearance | Off-white solid. |
| Solubility | Soluble in DMSO, methanol, water, dichloromethane or acetone. |
| Identity | Determined by 1H-NMR and MS. |
| Declaration | Manufactured by BioViotica. |
| InChi Key | WHIKSLGSXKIHCA-IGCCMALHSA-N |
| Smiles | CC\C(C)=C1/OC(=O)[C@H](C)[C@H](O)[C@H](CC2=CC=CN=C2)NC(=O)[C@@H](NC(=O)C2=C(O)C=CC=N2)[C@@H](C)OC1=O |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | +4°C |
| Handling Advice | Protect from light when in solution. |
| Use/Stability | Stable for at least 1 year after receipt when stored at +4°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
- Cyclodepsipeptide antibiotic. Unusual 12-membered macrocyclic depsipeptide comprises three unique subunits incorporating two substituted pyridines.
- Potent antitubercular agent.
- Inhibitor of NADH-dependent enoyl (Acyl-Carrier-Protein) reductase InhA, preventing mycolic acid synthesis in M. tuberculosis.
- Shown to be active against isoniazid-resistant mycobacteria.
Product References
- A new antibiotic, pyridomycin: K. Maeda, et al.; J. Antibiot. (Tokyo) 6, 140 (1953)
- Structure of pyridomycin: G. Koyama, et al.; Tetrahedron Lett. 37, 3587 (1967)
- Chemistry of pyridomycin: H. Ogawara, et al.; Chem. Pharm. Bull. 16, 679 (1968)
- Synthetic studies of pyridomycin. V. Total synthesis of pyridomycin: M. Kinoshita, et al.; Tetrahedron Lett. 30, 7419 (1989)
- Identification and characterization of the pyridomycin biosynthetic gene cluster of streptomyces pyridomyceticus NRRL B-2517: T. Huang, et al.; J. Biol. Chem. 286, 20648 (2011)
- Towards a new tuberculosis drug: pyridomycin - nature's isoniazid: R. C. Hartkoorn, et al.; EMBO Mol. Medicine 4, 1032 (2012)
- Pyridomycin bridges the NADH- and substrate-binding pockets of the enoyl reductase InhA: R. C. Hartkoorn, et al.; Nat. Chem. Biol. 10, 96 (2014)