Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
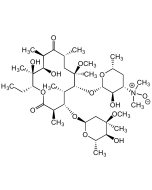
Josamycin

| Product Details | |
|---|---|
| Synonyms | Kitasamycin A3; Leucomycin A3; Turimycin A5 |
| Product Type | Chemical |
| Properties | |
| Formula |
C42H69NO15 |
| MW | 827.99 |
| CAS | 16846-24-5 |
| RTECS | OH4725810 |
| Source/Host Chemicals | Isolated from Streptomyces narbonensis var. josamyceticus var. nova. |
| Purity Chemicals | ≥90% (HPLC) |
| Appearance | White to off-white powder. |
| Solubility | Soluble in ethanol (25mg/ml), DMSO (15mg/ml) or DMF (25mg/ml). Sparingly soluble in water (0.05mg/ml). |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | XJSFLOJWULLJQS-LAEWXYAOSA-N |
| Smiles | CO[C@H]1[C@@H](CC(=O)O[C@H](C)C\C=C\C=C\[C@H](O)[C@H](C)CC(CC=O)[C@@H]1O[C@@H]1O[C@H](C)[C@@H](O[C@H]2C[C@@](C)(O)[C@@H](OC(=O)CC(C)C)[C@H](C)O2)C([C@H]1O)N(C)C)OC(C)=O |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | +4°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at +4°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Josamycin is a member of the leucomycin family of macrolide antibiotics produced by Streptomyces kitasatoensis. It is an antimicrobial against a wide variety of pathogens. It has activity against Gram-positive an Gram-negative bacteria. The mechanism of action is via inhibition of bacterial protein biosynthesis by binding reversibly to the subunit 50S of the bacterial ribosome, inhibiting peptidyltransferase and ribosomal translocation, thereby inhibiting translocation of peptidyl tRNA. Josamycin may overcome anticancer drug resistance by inhibiting the binding of vinblastine or cyclosporin A to P-glycoprotein (Pgp). It is used to study the modification of phagocytosis and cytokine production by macrolide antibiotics and immunomodulatory effects.
(1) K. Nitta, et al.; J. Antibiot. 20, 181 (1967) | (2) T. Osono, et al.; J. Antibiot. 20, 174 (1967) | (3) T. Bergan & B. Oydvin; Acta Pathol. Microbiol. Scand. B Microbiol. Immunol. 80, 101 (1972) | (4) E.L. Westerman, et al.; Antimicrob. Agents Chemother. 9, 988 (1976) | (5) R.E. Reese, et al.; Antimicrob. Agents Chemother. 10, 253 (1976) | (6) J.P. Guggenbichler, et al.; Infection 21, 259 (1993) | (7) K. Morikawa, et al.; Antimicrob. Agents Chemother. 38, 2643 (1994) | (8) T. Ono, et al.; Chemotherapy 42, 159 (1996) | (9) L. Wang, et al.; Clin. Exp. Pharmacol. Physiol. 27, 587 (2000) | (10) M. Lovmar, et al.; J. Biol. Chem. 279, 53506 (2004) | (11) E.Y. Choi, et al.; Biomed. Pharmacother. 105, 498 (2018)