Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
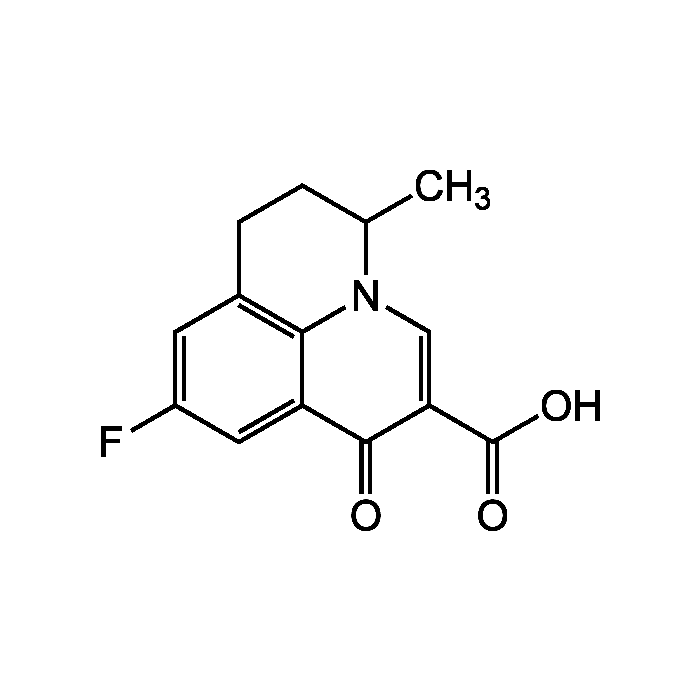
Flumequine
| Product Details | |
|---|---|
| Synonyms | 9-Fluoro-5-methyl-1-oxo-1,5,6,7-tetrahydropyrido[3,2,1-ij]quinoline-2-carboxylic acid |
| Product Type | Chemical |
| Properties | |
| Formula | C14H12FNO3 |
| MW | 261.3 |
| CAS | 42835-25-6 |
| RTECS | DK1672000 |
| Source/Host Chemicals | Synthetic. |
| Purity Chemicals | ≥97% (HPLC) |
| Appearance | White to off-white powder. |
| Solubility | Soluble in toluene. |
| Identity | Determined by NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | DPSPPJIUMHPXMA-UHFFFAOYSA-N |
| Smiles | CC1CCC2=C3N1C=C(C(O)=O)C(=O)C3=CC(F)=C2 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice |
Keep cool and dry. Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Flumequine is a fluoroquinolone synthetic chemotherapeutic antibiotic used to treat bacterial infections. Targets primarily gram negative bacteria, especially those which cause enteric infections in animals. It is used to study processes that affect mammalian chromosome and DNA unwinding at the level of gyrase/topoisomerases. It inhibits topoisomerases, which are needed for the transcription and replication of bacterial DNA. The inhibition of the topoisomerases results in strand breakage of the bacterial chromosome, supercoiling and resealing. Therefore, DNA replication and transcription is inhibited. It was also used to study hepatocarcinogenicity and DNA damage in mice and the mechanisms of quinolone resistance.
(1) G. Stilwell, et al.; Antimicrob. Agents Chemother. 7, 483 (1975) | (2) S.R. Rohlfing, et al.; J. Antimicrob. Chemother. 3, 615 (1977) | (3) M. Yoshida, et al.; Cancer Lett. 141, 99 (1999) | (4) A. Ruiz-Garcia, et al.; Eur. J. Pharm. Biopharm. 48, 253 (1999) | (5) Y. Kashida, et al.; Toxicol. Sci. 69, 317 (2002) | (6) Y. Kuroiwa, et al.; Arch. Toxicol. 81, 63 (2007)