Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Cellular Fibronectin (EDA) in Tumor Progression and Inflammation
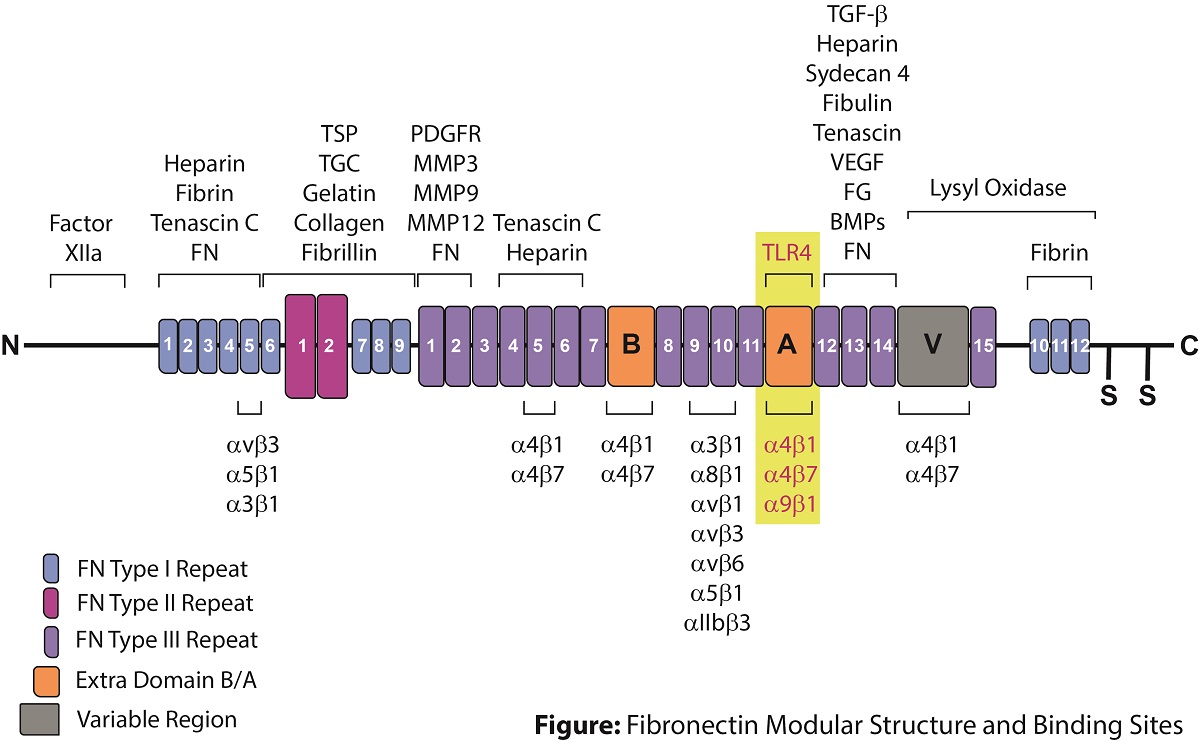
Fibronectin is a major component of the extracellular matrix that binds to integrins, cell surfaces and various compounds including collagen, fibrin, heparin, DNA and actin. It is found as two main forms: soluble plasma fibronectin (pFN) and cellular fibronectin (cFN) that is a component of the extracellular matrix (ECM). Fibronectin is produced by different types of cells (fibroblasts, smooth muscle cells, endothelial cells, platelets, and monocytes). Fibronectin is involved in cellular processes such as cell adhesion, migration, growth and survival, and it is required for embryonic development. pFN supports hemostasis and regulates thrombosis, accelerates healing by limiting the extent of inflammation. As a key component of a fibrin clot, pFN supports hemostasis by being rapidly deposited at the injured vessel wall and by supporting platelet aggregation via pFN-fibrin complexes. cFN forms and maintain tissue architecture and regulates cellular processes.
Fibronectin is encoded by a single gene, which generates 20 possible variants in humans through alternative splicing. Protein diversity is obtained through the inclusion or exclusion of a type III homology segment (extra domain A of FN (EDA) and extra domain B of FN (EDB)) and by the presence of a V region (IIICS), which can be assembled in 4 ways or completely excluded (see figure). Plasma fibronectin (pFN) lacks extra EDA and EDB segments.
The inclusion of Extra Domain A (EDA) in cellular fibronectin (cFN-EDA) is only expressed upon tissue damage and plays a major role in cell adhesion, growth, migration, tissue remodeling, fibroblast differentiation, inflammation, and it is important for processes such as wound healing and embryonic development. cFN-EDA is also associated with pathological processes such as atherosclerosis, lung fibrosis, liver fibrosis and tumor progression. cFN-EDA allows fibroblast differentiation by increasing α-smooth muscle actin (α-SMA) expression, collagen deposition, cell contractility and focal adhesion kinase (FAK) activation. cFN-EDA promotes cell spread by interacting with the integrins α5-β1, α4-β1 and α9-β1 and has been shown to promote angiogenesis, lymphangiogenesis and metastasis of colorectal tumors. cFN-EDA has also been used as a suitable antigen for CAR-T cell design. cFN-EDA also induces an inflammatory response in fibroblasts through binding to pattern recognition receptor TLR4, which regulates the NF-κB-dependent synthesis of cytokines, thus triggering immune responses.
Literature References:
-
Plasma and cellular fibronectin: distinct and independent functions during tissue repair: W.S. To & K.S. Midwood; Fibrogen. Tissue Rep. 4, 21 (2011)
-
Fibronectin and its soluble EDA-FN isoform as biomarkers for inflammation and sepsis: A. Lemanska-Perek & B. Adamik; Adv. Clin. Exp. Med. 28, 1561 (2019)
-
Shaping Up the Tumor Microenvironment With Cellular Fibronectin: G. Efthymiou, et al.; Front. Oncol. 10, 641 (2020)
-
Fibronectin in development and wound healing: J. Patten & K. Wang; Adv. Drug Deliv. Rev. 170, 353 (2021)
The STANDARD Antibody for Cellular Fibronectin (EDA) Research
anti-Fibronectin (EDA), mAb (blocking) (IST-9) (preservative free)
AG-20B-6001YPF (100µg, 500µg sizes & Bulk from Stock)
AdipoGen Life Sciences is a manufacturer of the antibody clone IST-9, the standard antibody to detect and study cellular Fibronectin-EDA (cFN-EDA) in Immunofluorescences (IHC, ICC) experiments. This antibody recognizes an epitope located in the Extra Domain A (EDA) sequence of human cellular fibronectin (FN) and does not detect plasma Fibronectin (pFN) lacking the EDA domain. The antibody has been shown to cross-react with mouse, rat, monkey, pig, dog, cow and chicken FN-EDA (according to literature).
The clone IST-9 is also a blocking antibody that inhibits the binding of Fibronectin EDA domain to α5-β1, α4-β1 and α9-β1 integrins and cell adhesion mediated by α5-β1, α4-β1 and α9-β1 integrins. The antibody has been shown to block the differentiation of fibroblasts into myo-fibroblasts and the TGF-β1-triggered enhancement of α-smooth muscle actin and collagen type I.
This antibody is available as a preservative version and in BULK quantities for long-term studies.
|
Product Specifications: Clone: IST-9
Contact us at info@adipogen.com and inquire about BULK Pricing! |
|
|
|
|
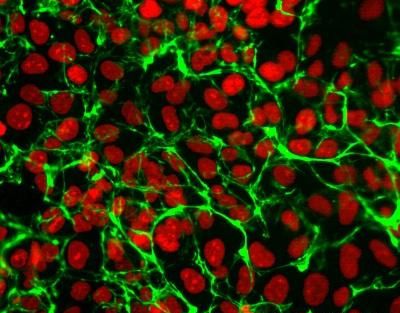
Figure: Immunocytochemical analysis of human esophageal epithelial cells using anti-Fibronectin (EDA), mAb (blocking) (IST-9) (preservative free) (AG-20B-6001YPF). An anti-mouse IgG secondary antibody was used. Nuclei were counterstained with propidium iodide.Figure: Immunocytochemical analysis of human esophageal epithelial cells using anti-Fibronectin (EDA), mAb (blocking) (IST-9) (preservative free) (AG-20B-6001YPF). An anti-mouse IgG secondary antibody was used. Nuclei were counterstained with propidium iodide. |
Selected Product References:
- Monoclonal antibodies in the analysis of fibronectin isoforms generated by alternative splicing of mRNA precursors in normal and transformed human cells: L. Borsi, et al.; J. Cell Biol. 104, 595 (1987)
- Localization of the cellular-fibronectin-specific epitope recognized by the monoclonal antibody IST-9 using fusion proteins expressed in E. coli: B. Carnemolla, et al.; FEBS Lett. 215, 269 (1987)
- Molecular and immunologic differences in canine fibronectins from articular cartilage and plasma: N. Burton-Wurster & G. Lust; Arch. Biochem. Biophys. 269, 32 (1989)
- Coordinate oncodevelopmental modulation of alternative splicing of fibronectin pre-messenger RNA at ED-A, ED-B, and CS1 regions in human liver tumors: F. Oyama, et al.; Cancer Res. 53, 2005 (1993)
- Endogenous fibronectin of blood polymorphonuclear leukocytes: immunochemical characterization and subcellular localization: R. Salcedo, et al.; Exp. Cell Res. 233, 25 (1997)
- The fibronectin domain ED-A is crucial for myofibroblastic phenotype induction by transforming growth factor-beta1: G. Serini, et al.; J. Cell Biol. 142, 873 (1998)
- Identification of two amino acids within the EIIIA (ED-A) segment of fibronectin constituting the epitope for two function-blocking monoclonal antibodies: Y.F. Liao, et al.; J. Biol. Chem. 274, 17876 (1999)
- Engagement of alpha4beta7 integrins by monoclonal antibodies or ligands enhances survival of human eosinophils in vitro: J. Meerschaert, et al.; J. Immunol. 163, 6217 (1999)
- Segmental antigen challenge increases fibronectin in bronchoalveolar lavage fluid: J. Meerschaert, et al.; Am. J. Respir. Crit. Care Med. 159, 619 (1999)
- Deletion of the alternatively spliced fibronectin EIIIA domain in mice reduces atherosclerosis: M.H. Tan, et al.; Blood 104, 11 (2004)
- Fibronectin-alpha4beta1 integrin interactions regulate metalloproteinase-9 expression in steatotic liver ischemia and reperfusion injury: C. Moore, et al.; Am. J. Pathol. 170, 567 (2007)
- Identification of the peptide sequences within the EIIIA (EDA) segment of fibronectin that mediate integrin alpha9beta1-dependent cellular activities: A.V. Shinde, et al.; J. Biol. Chem. 283, 2858 (2008)
- Fibronectin extra domain A (EDA) sustains CD133+/CD44+ subpopulation of colorectal cancer cells: J. Ou, et al.; Stem Cell Res. 11, 820 (2013)
- Irigenin, a novel lead from Western Himalayan chemiome inhibits Fibronectin-Extra Domain A induced metastasis in lung cancer cells: A. Amin, et al.; Sci. Rep. 6, 37151 (2016)
- Detection of soluble ED-A+ fibronectin and evaluation as novel serum biomarker for cardiac tissue remodeling: B. Ziffels, et al.; Dis. Mark. 2016, ID3695454 (2016)
- Cardiac inducing colonies halt fibroblast activation and induce cardiac/endothelial cells to move and expand via paracrine signaling: S. Mahapatra, et al.; Mol. Biol. Cell 33, ar96 (2022)
