Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Salinosporamide A [Marizomib] - Potent Proteasome Inhibitor
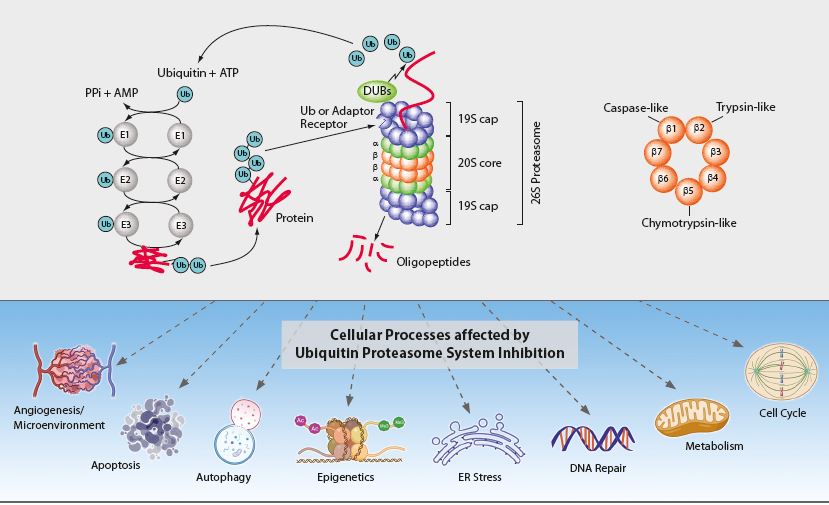
The ubiquitin-proteasome system (UPS) and the autophagic-lysosomal pathway are the two major degradation systems for both native and misfolded proteins in eukaryotic cells. They do not act independently from each other. Defective autophagy results in the accumulation of ubiquitinated proteins, impacting the flux of the UPS, while dysfunction of the UPS can promote a compensatory induction of autophagy. Through protein degradation and the maintenance of protein homeostasis, the UPS regulates many normal cellular processes including signal transduction, cell cycle control, transcription and apoptosis (see Figure). The regulated proteolysis of bulk and misfolded proteins is strictly controlled by the 26S proteasome complex.
Figure: The Ubiquitin-Proteasome System Inhibition and affected Cellular Processes
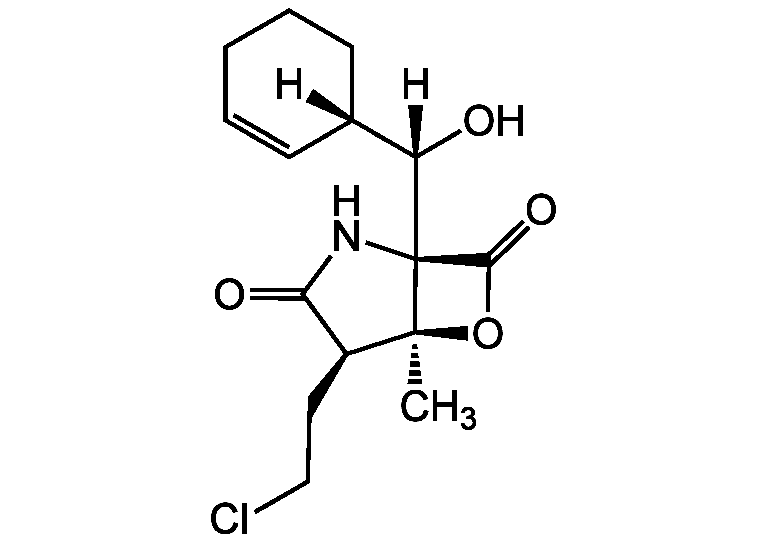
Salinosporamide A [Marizomib; MRZ]
The 26S proteasome complex recognizes polyubiquitinated proteins, which were marked for elimination by the E1, E2 and E3 ubiquitinating enzymes (see Figure). Upon recognition, unfolding and transfer of the de-ubiquitinated target protein by the 19S regulatory cap into the interior of the cylindrical 20S proteasome core particle, protein degradation is facilitated by catalytic β-subunits having nucleophilic N-terminal threonine (Thr1) residues. Although eukaryotic 20S proteasomes harbor seven different β-subunits in their two-fold symmetrical α7β7β7α7 stacked complexes, only three β-subunits per β-ring [subunits β1 (caspase-like), β2 (trypsin-like) and β5 (chymotrypsin-like)] are proteolytically active. These three β-subunits are major targets for small molecule proteasome inhibitors.
Salinosporamide A is a novel, second-generation irreversible pan-proteasome inhibitor, characterized by a unique β-lactone γ-lactam bicyclic ring structure. It inhibits the 3 constitutive activities of the mammalian 20S proteasome (chymotrypsin-like, β5 [CT-L]); trypsin-like, β2 [T-L] and caspase-like, β1 [C-L]). Salinosporamide A is effective at nanomolar concentrations in vitro and demonstrates penetration in vivo into multiple different organs including the CNS, crossing the blood-brain barrier (BBB). Non-clinical studies have demonstrated that salinosporamide A rapidly forms a covalent chemical bond at the active enzymatic sites (β5, β2 and β1) in the proteasome resulting in irreversible inhibition at nanomolar concentrations. Extensive preclinical studies demonstrate activity of salinosporamide A in a wide variety of hematopoietic, solid and CNS tumor models. Because of enhanced CNS penetrating capability of salinosporamide A, given its lipophilic structure, it is especially of interest as a reagent in CNS tumor research.
Literature References:
-
Salinosporamide A: a highly cytotoxic proteasome inhibitor from a novel microbial source, a marine bacterium of the new genus salinospora: R.H. Feling, et al.; Angew. Chem. Int. Ed. Engl. 42, 355 (2003)
-
Discovery and development of the anticancer agent salinosporamide A (NPI-0052): W. Fenical, et al.; Bioorg. Med. Chem. 17, 2175 (2009)
-
Generating a generation of proteasome inhibitors: from microbial fermentation to total synthesis of salinosporamide a (marizomib) and other salinosporamides: B.C. Potts & K.S. Lam; Mar. Drugs 8, 835 (2010) (Review)
-
Salinosporamide natural products: Potent 20S proteasome inhibitors as promising cancer chemotherapeutics: T.A. Gulder & B.S. Moore; Angew. Chem. Int. Ed. Engl. 49, 9346 (2010) (Review)
-
Marizomib, a proteasome inhibitor for all seasons: preclinical profile and a framework for clinical trials: B.C. Potts, et al.; Curr. Cancer Drug Targets 11, 254 (2011)
-
Proteasome Inhibition in Multiple Myeloma: Head-to-Head Comparison of Currently Available Proteasome Inhibitors: A. Besse, et al.; Cell Chem. Biol. 26, 340 (2019)
Salinosporamide A
Salinosporamide A is a potent, irreversible inhibitor of all the 3 proteolytic activities of the mammalian 20S proteasome. β5 subunit: chymotrypsin-like (EC50 = 3.5nM); β2 subunit: trypsin-like (EC50 = 28nM); β1 subunit: caspase-like or peptidyl-glutamyl peptide-hydrolyzing (PGPH) (EC50 = 430nM). Salinosporamide A is a potent anticancer compound. It triggers apoptosis with distinct proteasome activity and mechanism of action compared to bortezomib (Velcade) (Prod. No. AG-CR1-3602). It displays a longer inhibition duration than bortezomib and shows potent antileukemic activity against bortezomib-resistant leukemia cells.
AG-CN2-0444 (100µg, 1 mg, 5 mg, 50 mg and BULK)
AdipoGen Life Sciences is an original Manufacturer of high-purity Salinosporamide A. BULK quantities are available from Stock!
|
Product Specifications: CAS: 437742-34-2Source: Isolated from Salinospora tropical (Marine) Purity: >98% HPLC Identity: Determined by 1H-NMR Contact us at info@adipogen.com and inquire about BULK Pricing! |
 |
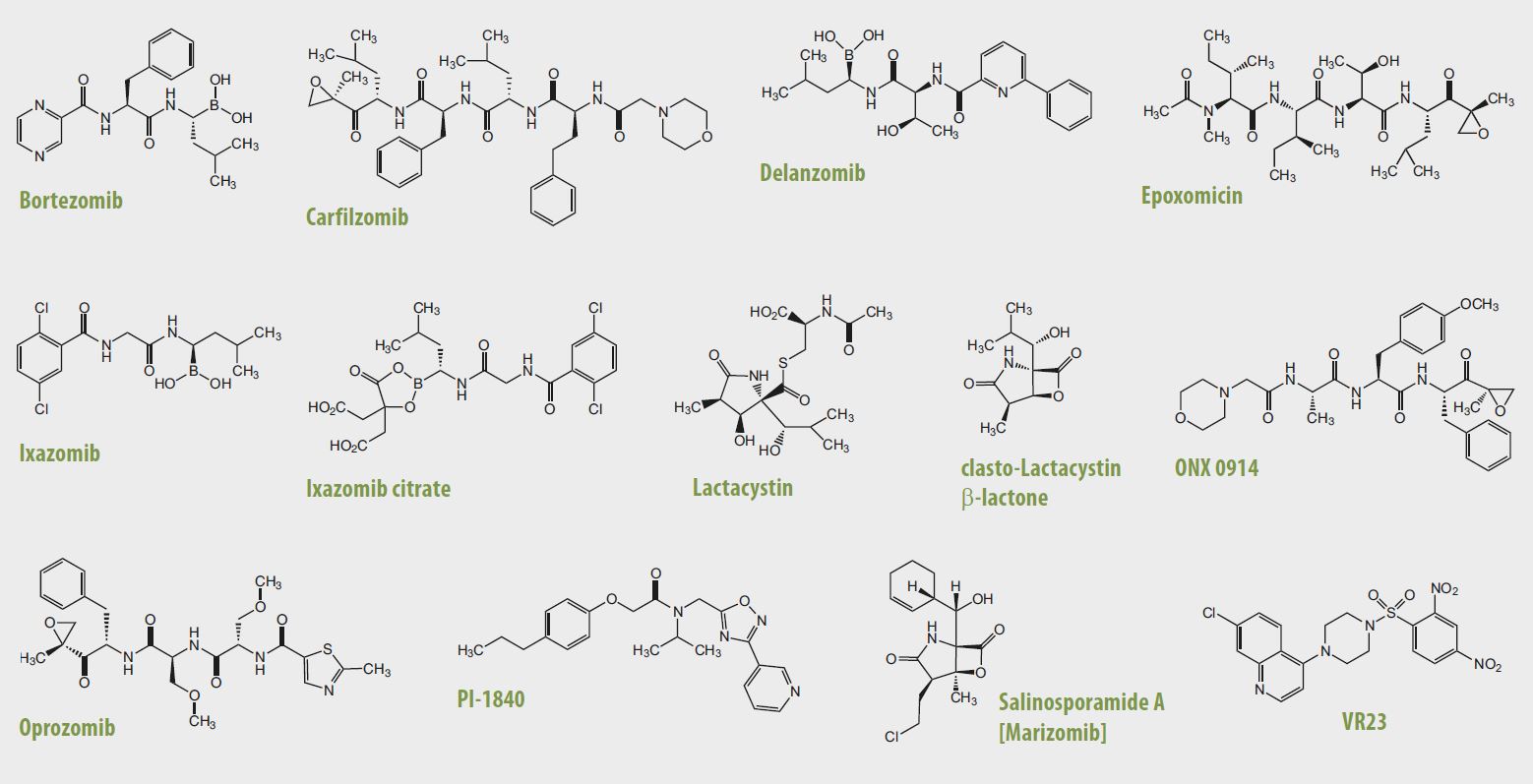
| Product Name | PID | Product Description |
| Bortezomib [PS-341] | AG-CR1-3602 | Inhibits chymotrypsin-like and caspase-like activity (IC50=3-5nM). |
| Carfilzomib [PR-171] | AG-CR1-3669 | Inhibits the chymotrypsin-like β5 subunit of the constitutive 20S proteasome (IC50=5.2nM) and the β5i subunit [LMP7] of the 20S immunoproteasome (IC50=14nM). |
| Delanzomib [CEP-18770] | AG-CR1-3673 | Inhibits the chymotrypsin-like β5 subunit of the constitutive 20S proteasome (IC50=3.8nM) and the caspase-like β1 subunit (IC50=~70nM). |
| (-)-Epigallocatechin gallate | AG-CN2-0063 | Inhibits chymotrypsin-like activity (IC50~200nm). |
| Epoxomicin | AG-CN2-0422 | Inhibits predominantly chymotrypsin-like activity (IC50=4nM). |
| Ixazomib [MLN2238] | AG-CR1-3670 | Inhibits all the three catalytic activities of the constitutive 20S proteasome: chymotrypsin-like β5 subunit (IC50=3.4nM), trypsin-like β2 subunit (IC50=3.5μM) and the caspase-like β1 subunit (IC50=0.03μM). |
| Ixazomib citrate [MLN9708] | AG-CR1-3671 | Inhibits all the three catalytic activities of the constitutive 20S proteasome: chymotrypsin-like β5 subunit (IC50=3.4nM), trypsin-like β2 subunit (IC50=3.5μM) and the caspase-like β1 subunit (IC50=0.03μM). |
| clasto-Lactacystin β-lactone | AG-CN2-0442 | Chymotrypsin-like, trypsin-like and caspase-like activity inhibitor (IC50~1μM). |
| Lactacystin | AG-CN2-0104 | Chymotrypsin-like, trypsin-like and caspase-like activity inhibitor (IC50=4.8μM). |
| ONX 0914 | AG-CR1-3674 | Inhibits the β5i subunit [LMP7] of the 20S immunoproteasome (IC50=73nM) with minimal cross-reactivity to the chymotrypsin-like β5 subunit of the constitutive 20S proteasome (IC50=1.04μM). |
| Oprozomib [ONX 0912] | AG-CR1-3672 | Inhibits the chymotrypsin-like β5 subunit of the constitutive 20S proteasome (IC50=36nM) and the β5i subunit [LMP7] of the 20S immunoproteasome (IC50=82nM). |
| PI-1840 [Proteasome Inhibitor] | AG-CR1-3675 | Inhibits the chymotrypsin-like β5-subunit of the constitutive 20S proteasome (IC50=27nM), with minimal trypsin-like (β2) and caspase-like (β1) activity (IC50= >100μM, for both). |
| Piperlongumine | AG-CN2-0024 | Inhibits the β5i subunit (LMP7) (IC50=15μM) with minimal inhibition of the human constitutive 20S proteasome. |
| Salinosporamide A [Marizomib] | AG-CN2-0444 | Inhibits all the three catalytic activities of the constitutive 20S proteasome: chymotrypsin-like (IC50=3.5nm); trypsin-like (IC50=28nm); caspase-like (IC50=430nm). |
| VR23 [Proteasome Inhibitor] | AG-CR1-3676 | Inhibits all the three catalytic activities of the constitutive 20S proteasome: chymotrypsin-like (IC50=50-100nm); trypsin-like (IC50=1nm); caspase-like (IC50=3μm). |
| Z-Leu-Leu-Phe-CHO [MG-110] | AG-CP3-0021 | Chymotrypsin-like activity inhibitor. |
| Z-Leu-Leu-Nva-CHO [MG-115] | AG-CP3-0015 | Chymotrypsin-like activity inhibitor. |
| Z-Leu-Leu-Leu-CHO [MG-132] | AG-CP3-0011 | Chymotrypsin-like and caspase-like activity inhibitor (IC50~1μM). |
| Z-Leu-Leu-Leu-B(OH)2 [MG-262] | AG-CP3-0024 | Chymotrypsin-like and caspase-like activity inhibitor (IC50~150nM). |