Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
SynKinase
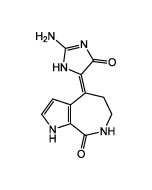
SAR-020106
As low as
CHF 0.00
In stock
Only %1 left
SYN-1189-M0011 mgCHF 270.00
SYN-1189-M0055 mgCHF 546.00
SYN-1189-M01010 mgCHF 858.00
SYN-1189-M100100 mgINQ
SYN-1189-M05050 mgINQ

| Product Details | |
|---|---|
| Synonyms | SAR020106 |
| Product Type | Chemical |
| Properties | |
| Formula | C19H19ClN6O |
| MW | 382.9 |
| CAS | 1184843-57-9 |
| Purity Chemicals | ≥95% |
| Appearance | Solid. |
| Solubility | Soluble in DMSO. |
| Declaration | Manufactured by SynKinase. |
| Other Product Data |
Target: EGFR - HER2 | Kinase Group: RTK | Substrate: Tyrosine Click here for Original Manufacturer Product Datasheet Our product description may differ slightly from the original manufacturers product datasheet. |
| InChi Key | SRBJWIBAMIKCMV-GFCCVEGCSA-N |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | +4°C |
| Use/Stability | Stable for at least 1 year after receipt when stored at +4°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
SAR-020106 is an ATP-competitive, potent, and selective CHK1 inhibitor with an IC(50) of 13.3nM on the isolated human enzyme. This compound abrogates an etoposide-induced G(2) arrest with an IC(50) of 55nM in HT29 cells, and significantly enhances the cell killing of gemcitabine and SN38 by 3.0- to 29-fold in several colon tumor lines in vitro and in a p53-dependent fashion. Biomarker studies have shown that SAR-020106 inhibits cytotoxic drug-induced autophosphorylation of CHK1 at S296 and blocks the phosphorylation of CDK1 at Y15 in a dose-dependent fashion both in vitro and in vivo.
Product References
- The preclinical pharmacology and therapeutic activity of the novel CHK1 inhibitor SAR-020106: M.I. Walton, et al.; Mol. Cancer Ther. 9, 89 (2010)
- The Chk1 inhibitor SAR-020106 sensitizes human glioblastoma cells to irradiation, to temozolomide, and to decitabine treatment: I. Patties, et al.; J. Exp. Clin. Cancer Res. 38, 420 (2019)