Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
BioViotica
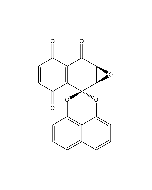
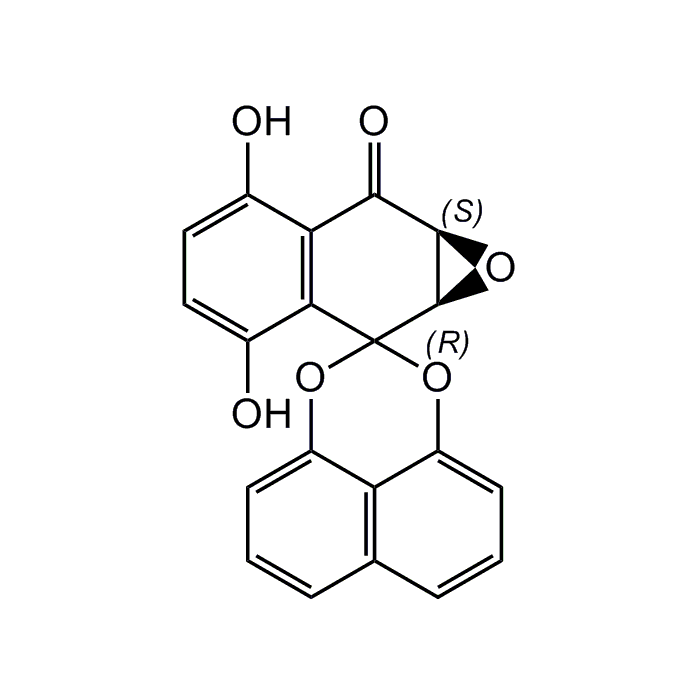
Palmarumycin C3
As low as
155
CHF
CHF 155.00
In stock
Only %1 left
BVT-0078-M0011 mgCHF 155.00
BVT-0078-M0055 mgCHF 470.00
| Product Details | |
|---|---|
| Synonyms | (1a'R,7a'S)-3',6'-Dihydroxy-1a'H-spiro[naphtho[1,8-de][1,3]dioxine-2,2'-naphtho[2,3-b]oxiren]-7'(7a'H)-one |
| Product Type | Chemical |
| Properties | |
| Formula |
C20H12O6 |
| MW | 348.3 |
| CAS | 159934-11-9 |
| Source/Host Chemicals | Isolated from Sphaeropsidales sp. |
| Purity Chemicals | ≥97% (HPLC) |
| Appearance | Yellow to green solid. |
| Solubility | Soluble in DMSO, dichloromethane, acetone or methanol. |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by BioViotica. |
| InChi Key | BDXBHYOFNNANPN-RTBURBONSA-N |
| Smiles | OC1=CC=C(O)C2=C1C(=O)[C@H]1O[C@H]1C21OC2=CC=CC3=C2C(O1)=CC=C3 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Use/Stability |
Stable for at least 1 year after receipt when stored at -20°C. After reconstitution protect from light at -20°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
- Rasfarnesyltransferase inhibitor.
- Antifungal, antibacterial and herbicidal.
- Structurally unique natural products. The basic structure of palmarumycin C1 can be modified by a number of hydroxylation, oxygenation, dehydrogenation and chlorination steps.
- Antioxidant.
Product References
- Biologically Active Metabolites from Fungi, 4. Palmarumycins CP1-CP4 from Coniothyrium palmarum: Isolation, structure elucidation, and biological activity: K. Krohn, et al.; Liebigs Ann. Chem. 1994, 1093 (1994)
- Biologically Active Metabolites from Fungi, 5. Palmarumycins C1-C16 from Coniothyrium sp.: Isolation, structure elucidation, and biological activity: K. Krohn, et al.; Liebigs Ann. Chem. 1994, 1099 (1994)
- Secondary metabolites by chemical screening, 42 Cladospirones B to I from Sphaeropsidales sp. F-24'707 by variation of culture conditions: H.B. Bode, et al.; Eur. J. Org. Chem. 2000, 3185-3193 (2000)
- Unified route to the palmarumycin and preussomerin natural products. Enantioselective synthesis of (-)-preussomerin G: A.G. Barrett, et al.; J. Org. Chem. 67, 2735-2750 (2002)
- Natural products derived from naphthalenoid precursors by oxidative dimerization: K. Krohn; Prog. Chem. Org. Nat. Prod. 85, 1-49 (2003) (Review)
- Antimicrobial and Antioxidant Activities and Effect of 1-Hexadecene Addition on Palmarumycin C2 and C3 Yields in Liquid Culture of Endophytic Fungus Berkleasmium sp. Dzf12: Y. Mou, et al.; Molecules 18, 15587 (2013)