Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
BioViotica
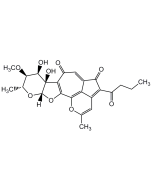
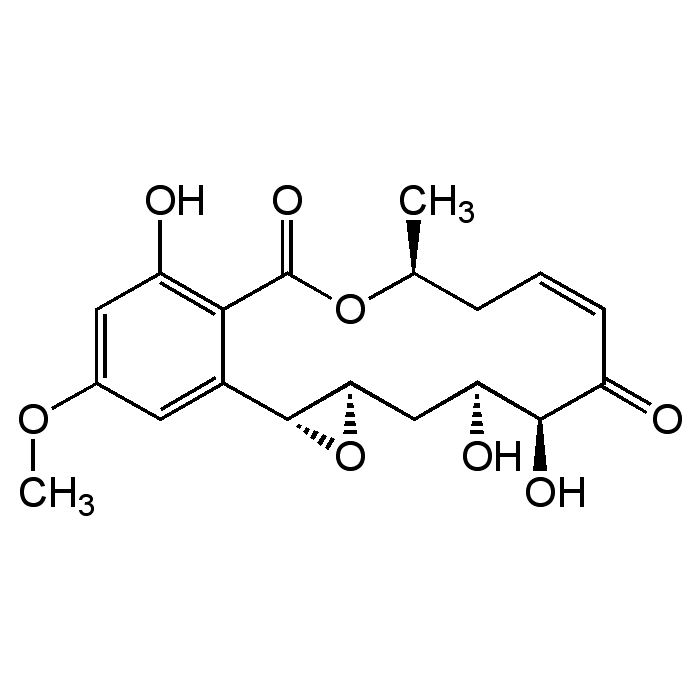
Hypothemycin
As low as
130
CHF
CHF 130.00
In stock
Only %1 left
BVT-0067-C250250 µgCHF 130.00
BVT-0067-M0011 mgCHF 395.00
| Product Details | |
|---|---|
| Product Type | Chemical |
| Properties | |
| Formula |
C19H22O8 |
| MW | 378.4 |
| CAS | 76958-67-3 |
| Source/Host Chemicals | Isolated from Phoma sp. |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | White to off-white solid. |
| Solubility | Soluble in DMSO or acetone; insoluble in methanol or water. |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by BioViotica. |
| InChi Key | SSNQAUBBJYCSMY-KNTMUCJRSA-N |
| Smiles | COC1=CC(O)=C2C(=C1)[C@H]1O[C@@H]1C[C@H](O)[C@H](O)C(=O)\C=C/C[C@H](C)OC2=O |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Use/Stability |
Stable for at least 1 year after receipt when stored at -20°C. After reconstitution protect from light at -20°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
- Antifungal.
- Cytotoxic against some tumor cell lines, partly attributed to inhibition of Ras-inducible genes.
- Inhibits proliferation of mouse and human T cells.
- Modulates production of cytokines during T cell activation.
- Facilitates the ubiquitinylation process of cyclin D1.
- Potent and selective threonine/tyrosine-specific kinase, MEK and other protein kinases inhibitor in both in vitro and in vivo studies.
Product References
- Metabolites of pyrenomycetes XIII: Structure of (+) hypothemycin, an antibiotic macrolide from hypomyces trichothecoides: M.S.R. Nair & S.T. Carey; Tetrahedron Lett. 21, 2011 (1980)
- Metabolites of pyrenomycetes. XIV: Structure and partial stereochemistry of the antibiotic macrolides hypothemycin and dihydrohypothemycin: M.S.R. Nair, et al.; Tetrahedron 37, 2445 (1981)
- Revised structure and stereochemistry of hypothemycin: T. Agatsuma et al.; Chem. Pharm. Bull. 41, 373 (1993)
- Antitumor efficacy of hypothemycin, a new Ras-signaling inhibitor: H. Tanaka, et al.; Jpn. J. Cancer Res. 90, 1139 (1999)
- Hypothemycin inhibits the proliferative response and modulates the production of cytokines during T cell activation: R. Camacho, et al.; Immunopharmacology 44, 255 (1999)
- Resorcylic acid lactones: naturally occurring potent and selective inhibitors of MEK: A. Zhao, et al.; J. Antibiot. 52, 1086 (1999)
- Suppression of oncogenic transformation by hypothemycin associated with accelerated cyclin D1 degradation through ubiquitin-proteasome pathway: H. Sonoda, et al.; Life Sci. 65, 381 (1999)
- Targeted covalent inactivation of protein kinases by resorcylic acid lactone polyketides: A. Schirmer, et al.; PNAS 103, 4234 (2006)
- Chemistry and biology of resorcylic acid lactones: N. Winssinger, et al.; Chem. Commun. (Camb). 1, 22 (2007), (Review)
- The resorcyclic acid lactone Hypothemycin selectively inhibits the mitogen-activated protein kinase kinase-extracellular signal-regulated kinase pathway in cells: H. Fukazawa, et al.; Biol. Pharm. Bull. 33, 168 (2010)