Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
AdipoGen Life Sciences
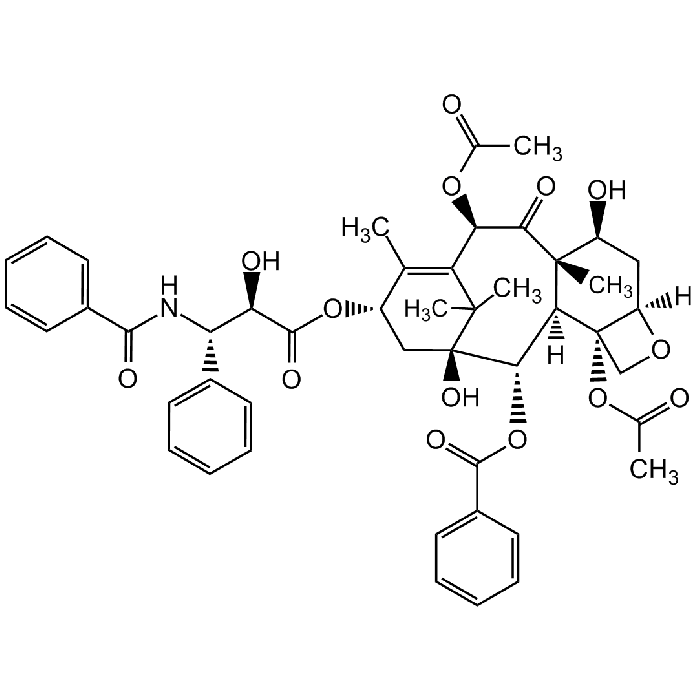
Paclitaxel
As low as
25
CHF
CHF 25.00
In stock
Only %1 left
AG-CN2-0045-M0011 mgCHF 25.00
AG-CN2-0045-M0055 mgCHF 40.00
AG-CN2-0045-M02525 mgCHF 80.00
AG-CN2-0045-M100100 mgCHF 220.00
| Product Details | |
|---|---|
| Synonyms | Taxol; BMS 181339-01; NSC 125973, PTX |
| Product Type | Chemical |
| Properties | |
| Formula |
C47H51NO14 |
| MW | 853.9 |
| Merck Index | 14: 6982 |
| CAS | 33069-62-4 |
| Source/Host Chemicals | Isolated from the bark of the pacific yew tree (Taxus brevifolia). |
| Purity Chemicals | ≥99% (HPLC) |
| Appearance | White powder. |
| Solubility | Soluble in DMSO, ethanol or acetonitrile. |
| Identity | Determined by 1H-NMR. |
| InChi Key | RCINICONZNJXQF-MZXODVADSA-N |
| Smiles | [H][C@@]12C[C@H](O)[C@@]3(C)C(=O)[C@H](OC(C)=O)C4=C(C)[C@H](C[C@@](O)([C@@H](OC(=O)C5=CC=CC=C5)[C@]3([H])[C@@]1(CO2)OC(C)=O)C4(C)C)OC(=O)[C@H](O)[C@@H](NC(=O)C1=CC=CC=C1)C1=CC=CC=C1 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice | Keep cool and dry. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
- Anticancer compound [1, 11, 14].
- Chemotherapeutic used in patients with cancer and advanced forms of Kaposi's sarcoma [11, 12].
- Microtubule assembly stabilizer. Reversibly binds to polymerized tubulin [2, 3, 6, 13, 14].
- Anti-mitotic. Mitotic spindle assembly, chromosome segregation and cell division inhibitor. Induces cell cycle arrest at the G2/M phase [4, 5, 10, 13].
- Apoptosis inducer through aberrant activation of cyclin-dependent kinases (CKDs) and the c-Jun N-terminal kinase/stress activated protein kinase (JNK/SAPK) [7-9, 10].
- Immunosuppressor. Immunostimulant [15].
- Antiproliferative agent for the prevention of restenosis [16].
- TRAIL sensitizer [17]
Product References
- Cytotoxic studies of paclitaxel (Taxol®) in human tumour cell lines: J.E. Liebmann, et al.; Br. J. Cancer 68, 1104 (1993)
- Taxol (paclitaxel): mechanisms of action: S.B. Horwitz; Ann. Oncol. 5, S3 (1994) (Review)
- Taxol (paclitaxel): a novel anti-microtubule agent with remarkable anti-neoplastic activity: R. Foa, et al.; Int. J. Clin. Lab. Res. 24, 6 (1994) (Review)
- Taxol-induced mitotic block triggers rapid onset of a p53-independent apoptotic pathway: C.M. Woods, et al.; Mol. Med. 1, 506 (1995)
- Tubulin as a target for anticancer drugs: agents which interact with the mitotic spindle: A. Jordan, et al.; Med. Res. Rev. 18, 259 (1998)
- How Taxol stabilises microtubule structure: L.A. Amos & J. Löwe; Chem. Biol. 6, R65 (1999) (Review)
- Mechanisms of Taxol-induced cell death are concentration dependent: K. Torres & S.B. Horwitz; Cancer Res. 58, 3620 (1998)
- Molecular effects of paclitaxel: myths and reality (a critical review): M.V. Blagosklonny & T. Fojo; Int. J. Cancer 83, 151 (1999)
- Microtubule dysfunction induced by paclitaxel initiates apoptosis through both c-Jun N-terminal kinase (JNK) dependent and -independent pathways in ovarian cancer cells: T.H. Wang, et al.; J. Biol. Chem. 274, 8208 (1999)
- Paclitaxel-induced cell death: where the cell cycle and apoptosis come together: T.H. Wang, et al.; Cancer 88, 2619 (2000)
- Paclitaxel in cancer therapy: T.M. Mekhail & M. Markman; Expert Opin. Pharmacother. 3, 755 (2002)
- Overcoming multidrug resistance in taxane chemotherapy: R. Geney, et al.; Clin. Chem. Lab. Med. 40, 918 (2002)
- Taxanes: microtubule and centrosome targets, and cell cycle dependent mechanisms of action: M. Abal, et al.; Curr. Cancer Drug Targets 300, 193 (2003)
- Microtubule-stabilizing natural products as promising cancer therapeutics: B.M. Gallagher Jr.; Curr. Med. Chem. 14, 2959 (2007)
- Paclitaxel and immune system: A. Javeed, et al.; Eur. J. Pharm. Sci. 38, 283 (2009) (Review)
- Anti-proliferative compounds for the prevention of restenosis: M.C. Lavigne; Curr. Pharm. Des. 16, 3989 (2010)
- Novel HTS strategy identifies TRAIL-sensitizing compounds acting specifically through the Caspase-8 apoptotic axis: D. Finlay, et al.;PLoS One 5, e13375 (2010)
- Eribulin induces micronuclei and enhances the nuclear localization of cGAS in triple-negative breast cancer cells: H. Yamada, et al.; Res. square ahead of print (2023)