Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
BioViotica
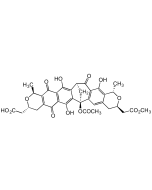
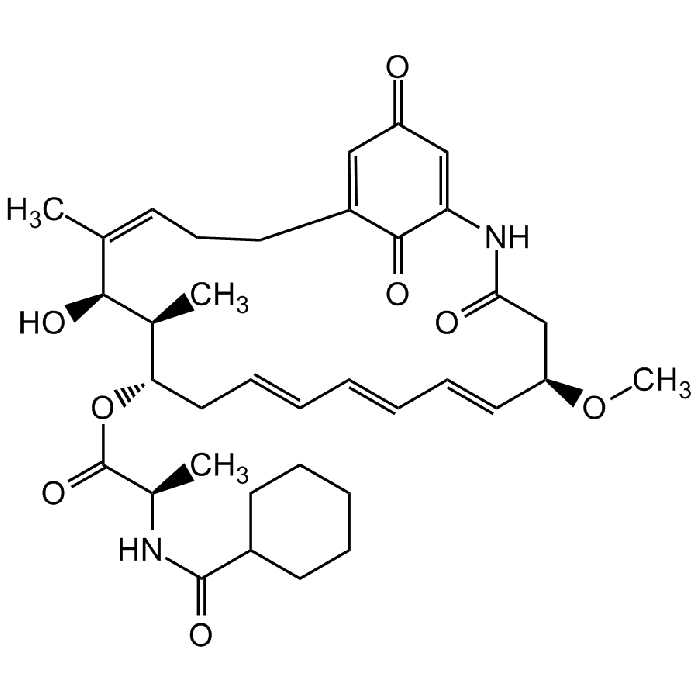
Ansatrienin A
As low as
110
CHF
CHF 110.00
In stock
Only %1 left
BVT-0246-M0011 mgCHF 110.00
| Product Details | |
|---|---|
| Synonyms | Mycotrienin I; Antibiotic T 23I |
| Product Type | Chemical |
| Properties | |
| Formula |
C36H48N2O8 |
| MW | 636.8 |
| CAS | 82189-03-5 |
| RTECS | AY4553220 |
| Source/Host Chemicals | Isolated from Streptomyces collinus. |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | Yellow powder. |
| Solubility | Soluble in 100% ethanol, methanol, dimethyl formamide or DMSO. |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by BioViotica. |
| InChi Key | WWUVMHRJRCRFSL-UOZMSBJPSA-N |
| Smiles | CO[C@@H]1CC(=O)NC2=CC(=O)C=C(CC\C=C(C)/[C@H](O)[C@@H](C)[C@H](C\C=C\C=C\C=C\1)OC(=O)[C@@H](C)NC(=O)C1CCCCC1)C2=O |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice | Protect from light when in solution. |
| Use/Stability |
Stable for at least 1 year after receipt when stored at -20°C. After reconstitution protect from light at -20°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
- Antibiotic.
- Antitumor compound. Active against several cell lines. Shown to potentiate several clinical anti-cancer agents.
- Inhibits TNF-α-induced expression of ICAM-1 (IC50=570nM).
- Antifungal.
- Inhibits osteoclastic bone resorption.
Product References
- Mycotrienin, a new polyene antibiotic isolated from Streptomyces: C. Coronelli, et al.; J. Antibiot. (Tokyo) 20, 329 (1967)
- Die Konstitution der fungistatischen Ansamycin-Antibiotica Ansatrienin A und B: M. Damberg, et al.; Tetrahedron Lett. 23, 59 (1982)
- Studies on mycotrienin antibiotics, a novel class of ansamycins. I. Taxonomy, fermentation, isolation and properties of mycotrienins I and II: M. Sugita, et al.; J. Antibiot. (Tokyo) 35, 1460 (1982)
- Studies on mycotrienin antibiotics, a novel class of ansamycins. II. Structure elucidation and biosynthesis of mycotrienins I and II: M. Sugita, et al.; J. Antibiot. (Tokyo) 35, 1467 (1982)
- Potentiation of mitomycin C, 6-mercaptopurine, bleomycin, cis-diamminedichloroplatinum and 5-fluorouracil by mycotrienins and mycotrienols: M. Kuwano, et al.; Gann. 74, 759 (1983)
- Mycotrienins. A new class of potent inhibitors of osteoclastic bone resorption: D. Feuerbach, et al.; J. Biol. Chem. 270, 25949 (1995)
- (+)-trienomycin A, B, C, and F and (+)-mycotrienins I and II: relative and absolute stereochemistry: A. B. Smith, et al.; JACS 118, 8308 (1996)
- Total syntheses of (+)-trienomycins A and F via a unified strategy: A. B. Smith, et al.; JACS 118, 8316 (1996)
- Total synthesis of (+)-mycotrienol and (+)-mycotrienin I: application of asymmetric crotylsilane bond constructions: C. E. Masse, et al.; JACS 120, 4123-4134 (1998)
- Mycotrienin II, a translation inhibitor that prevents ICAM-1 expression induced by pro-inflammatory cytokines: Y. Yamada, et al.; J. Antibiot. 64, 361 (2011)