Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
BioViotica
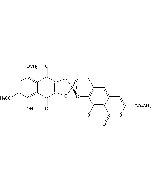
γ-Rubromycin
95
CHF
CHF 95.00
In stock
BVT-0007-M0011 mgCHF 95.00
BVT-0007-M0055 mgCHF 385.00

| Product Details | |
|---|---|
| Product Type | Chemical |
| Properties | |
| Formula |
C26H18O12 |
| MW | 522.4 |
| CAS | 27267-71-6 |
| Source/Host Chemicals | Isolated from Streptomyces sp. |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | Red powder. |
| Solubility | Soluble in methylene chloride, DMSO or methanol. |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by BioViotica. |
| InChi Key | CKLKRRFSZZUFKT-UHFFFAOYSA-N |
| Smiles | COC(=O)C1=CC2=CC3=C(O[C@@]4(CC5=C(O)C6=C(C(O)=C5O4)C(=O)C(OC)=CC6=O)CC3)C(O)=C2C(=O)O1 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice | Protect from light when in solution. |
| Use/Stability |
Stable for at least 1 year after receipt when stored at -20°C. After reconstitution protect from light at -20°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
- Antibiotic.
- HIV-1 reverse transcriptase and human telomerase inhibitor.
- Antibacterial.
- Anticancer agent. ytostatically active against different tumor cell lines.
- Human telomerase inhibitor.
Product References
- Rubromycins. 3. The constitution of alpha-rubromycin, beta-rubromycin, gamma-rubromycin, and gamma-iso-rubromycin: H. Brockmann & A. Zeeck; Chem. Ber. 103, 170 (1970)
- Inhibition of human immunodeficiency virus-1 reverse transcriptase activity by rubromycins: competitive interaction at the template.primer site: M.E. Goldman, et al.; Mol. Pharmacol. 38, 20 (1990)
- Inhibition of human telomerase by rubromycins: implication of spiroketal system of the compounds as an active moiety: T. Ueno, et al.; Biochemistry 39, 5995 (2000)
- Structural and biosynthetic investigations of the rubromycins: C. Puder, et al.; Eur. J. Org. Chem. 2000, 729 (2000)
- Determination of the absolute configurations of gamma-rubromycins and related spiro compounds: G. Bringmann, et. al.; Eur. J. Org. Chem. 2000, 2729 (2000)
- Rubromycins: Structurally intriguing, biologically valuable, synthetically challenging antitumour antibiotics: M. Brasholz, et al.; Eur. J. Org. Chem. 3801 (2007)
- Total synthesis of (+/-)-gamma-rubromycin on the basis of two aromatic Pummerer-type reactions: S. Akai, et al.; Angew. Chem. Int. Ed. 46, 7458 (2007)
- Interaction of heliquinomycin with single-stranded DNA inhibits MCM4/6/7 helicase: T. Sugiyama, et. al.; J. Biochem. 151, 129 (2012)
- A Convergent Total Synthesis of the Telomerase Inhibitor (±)-γ-Rubromycin: M. Wilsdorf & H.-U. Reißig; Angew. Chem. Int. Ed. 53, 4495 (2014)
- Isolation, biological activity, biosynthesis and synthetic studies towards the rubromycin family of natural products: D.J. Atkinson & M.A. Brimble; Nat. Prod. Rep. 32, 811 (2015)