Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
AdipoGen Life Sciences
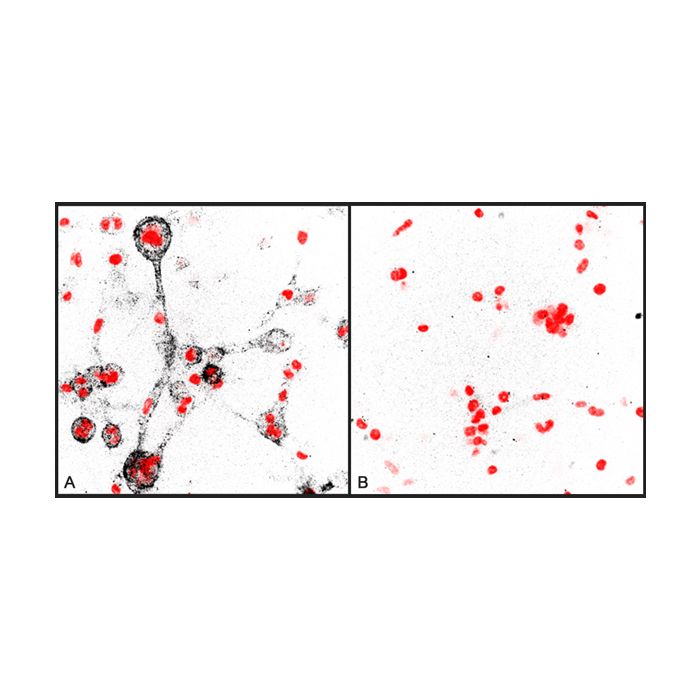
anti-Collagen Type 1 (3/4 fragment), pAb
| Product Details | |
|---|---|
| Synonyms | COL1; α-1 Type I Collagen |
| Product Type | Polyclonal Antibody |
| Properties | |
| Source/Host | Rabbit |
| Immunogen/Antigen | Synthetic peptide (human sequence) corresponding to the C-terminal end of the N-terminal three quarter collagen fragment (Col1 ¾). |
| Application |
Immunocytochemistry: (0.5-10μg/ml) |
| Crossreactivity |
Bovine Cat Chicken Cow Dog Guinea pig Hamster Human Mouse Pig Rat Sheep |
| Specificity |
Recognizes rat and bovine collagen type 1. Based on sequence identity should also recognize human, mouse, guinea pig, dog, cat, donkey, pig, cow, sheep and chicken collagen type 1. |
| Purity | Antigen affinity purified. |
| Concentration | 250μg/ml |
| Formulation | Liquid. In PBS with 1 mg/ml BSA and 0.02% sodium azide. |
| Isotype Negative Control | |
| Accession Number | UniProt ID P02452: Col1a (human) |
| Shipping and Handling | |
| Shipping | BLUE ICE |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice | Avoid freeze/thaw cycles. |
| Use/Stability | Stable for at least 1 year after receipt when stored at -20°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
The proteolysis of collagens plays an important role in numerous physiological and pathological situations such as morphogenesis, wound healing, arthritis, arteriosclerosis and tumor metastasis. Triple helical type I collagens are made up of two α1 (I) and one α2 (I) chains, and are found in skin, tendon, ligament and interstitial tissues. Due to their fibrillary structure native collagens are resistant to most proteases. They are substrates for certain matrix metalloproteinases (MMPs), which constitute a family of zinc-dependent enzymes catalyzing the degradation of extracellular matrix components. Initial MMP-8 dependent cleavage of collagen into the characteristic ¾ and ¼ fragments has been shown to enable MMP-9 diffusion along the protein helix with preferential binding to the collagen ¾ fragment tail. Finally, untwisting of the helix end results in the local denaturation of the triple helical structure.
- Endosomal WASH and exocyst complexes control exocytosis of MT1-MMP at invadopodia: P. Monteiro, et al.; J. Cell Biol. 203, 1063 (2013)
- Physical limits of cell migration: Control by ECM space and nuclear deformation and tuning by proteolysis and traction force: K. Wolf, et al.; J. Cell Biol. 201, 1069 (2013)
- Cell jamming: Collective invasion of mesenchymal tumor cells imposed by tissue confinement: A. Haeger, et al.; BBA 1840, 2386 (2014)
- Discoidin domain receptor 1 controls linear invadosome formation via a Cdc42–Tuba pathway: A. Juin, et al.; J. Cell Biol. 207, 517 (2014)
- Multiparametric Classification Links Tumor Microenvironments with Tumor Cell Phenotype: B. Gligorijevic, et al.; PLoS Biol. 12, e1001995 (2014)
- Diverse matrix metalloproteinase functions regulate cancer amoeboid migration: J.L. Orgaz, et al.; Nat. Commun. 5, 4255 (2014)
- ARF6–JIP3/4 regulate endosomal tubules for MT1-MMP exocytosis in cancer invasion: V. Marchesin, et al.; J. Cell Biol. 211, 339 (2015)
- Flightless I interacts with NMMIIA to promote cell extension formation, which enables collagen remodeling: P.D. Arora, et al.; Mol. Biol. Cell 26, 2279 (2015)
- Filamin A regulates the organization and remodeling of the pericellular collagen matrix: M. Mezawa, et al.; FASEB J. 30, 3613 (2016) (Western Blot)
- p63/MT1-MMP axis is required for in situ to invasive transition in basal-like breast cancer: C. Lodillinsky, et al. Oncogene 35, 344 (2016)
- PI3Kβ links integrin activation and PI(3,4)P2 production during invadopodial maturation: Z. Erami, et al.; Mol. Biol. Cell. 30, 2367 (2019)
- Decreased levels of endocytic collagen receptor Endo180 in dermal fibroblasts lead to decreased production of type I collagen and increased expression of matrix metalloproteinase-1: H. Iwahashi, et al.; Photodermatol. Photoimmunol. Photomed. 38, 150 (2021)
- Intermediate filaments promote glioblastoma cell invasion by controlling cell deformability and mechanosensitive gene expression: S. Etienne-Manneville, et al.; Res. Square ahead of print (2023)





