Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
AdipoGen Life Sciences
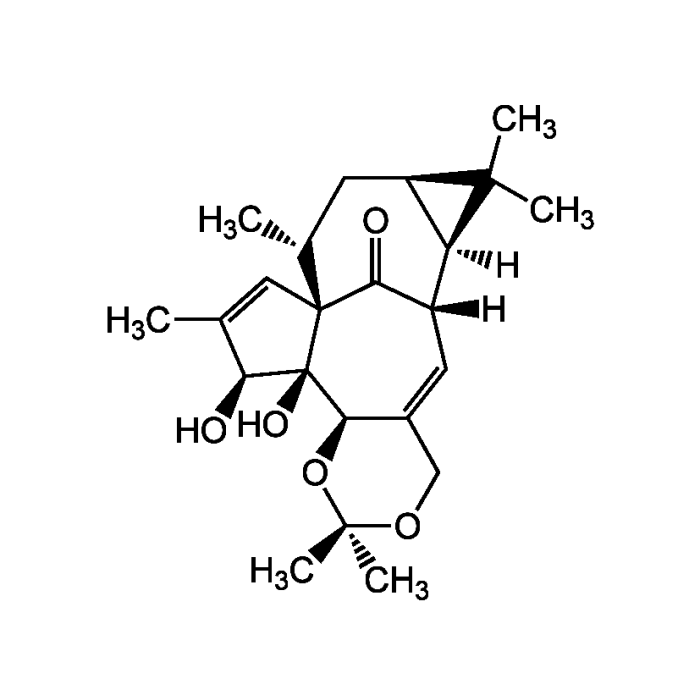
Ingenol-5,20-acetonide
As low as
70
CHF
CHF 70.00
In stock
Only %1 left
AG-CN2-0029-M0011 mgCHF 70.00
AG-CN2-0029-M0055 mgCHF 280.00
| Product Details | |
|---|---|
| Product Type | Chemical |
| Properties | |
| Formula |
C23H32O5 |
| MW | 388.5 |
| CAS | 77573-43-4 |
| Source/Host Chemicals | Semisynthetic. |
| Purity Chemicals | ≥95% (HPLC) |
| Appearance | White solid. |
| Solubility | Soluble in methanol, DMSO or dichloromethane. |
| InChi Key | ONMDPPVVEFWDOD-DVBBJZJXSA-N |
| Smiles | [H][C@@]12[C@@H](C[C@@H](C)[C@]34C=C(C)[C@H](O)[C@@]3(O)[C@@H]3OC(C)(C)OCC3=C[C@]1([H])C4=O)C2(C)C |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Use/Stability | Stable for at least 1 year after receipt when stored at -20°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
- Intermediate and starting material for the synthesis and preparation of ingenol 3-ester derivatives.
- For use in chemical synthesis see references.
- Has improved shelf life compared to ingenol.
Product References
- The chemistry of ingenol. 1. Ingenol and some of its derivatives: J.H. Opferkuch, et al.; Z. Naturforsch. 36b, 878 (1981)
- Synthesis of Modified Ingenol Esters: G. Appendino, et al.; Eur. J. Org. Chem. 1999, 3413 (1999)
- Ingenol esters induce apoptosis in Jurkat cells through an AP-1 and NF-kappaB independent pathway: M. Blanco-Molina, et al.; Chem. Biol. 8, 767 (2001)
- Synthesis of sapintoxin D and N-methylanthranilate-based fluorescent bioprobes: F. Mainieri, et al.; Nat. Prod. Commun. 2, 375 (2007)





