Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
AdipoGen Life Sciences
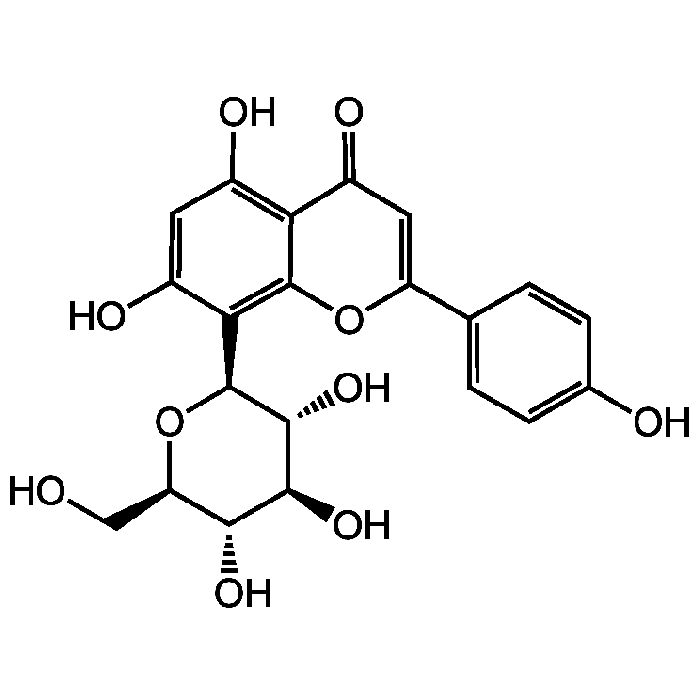
Vitexin
As low as
40
CHF
CHF 40.00
In stock
Only %1 left
AG-CN2-0425-M0055 mgCHF 40.00
AG-CN2-0425-M02525 mgCHF 130.00
| Product Details | |
|---|---|
| Synonyms | 8-Glucopyranosylapigenin; 8-Glucosylapigenin; Orientoside |
| Product Type | Chemical |
| Properties | |
| Formula |
C21H20O10 |
| MW | 432.4 |
| CAS | 3681-93-4 |
| RTECS | DJ2984000 |
| Source/Host Chemicals | Isolated from Crataegus pinnatifida. |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | Yellow crystalline powder. |
| Solubility | Soluble in DMSO or dimethylformamide. |
| InChi Key | SGEWCQFRYRRZDC-VPRICQMDSA-N |
| Smiles | OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)C1=C2OC(=CC(=O)C2=C(O)C=C1O)C1=CC=C(O)C=C1 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice |
Keep cool and dry. Protect from light. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
- α-Glucosidase inhibitor [3, 8].
- Antioxidant [1, 3].
- HIF-1 α inhibitor [2].
- Anti-metastatic [2, 13].
- Apoptosis inducer [4, 9, 13].
- Tumor suppressor [4, 9].
- Adipogenesis inhibitor [5].
- Inhibits SIRT6 in vitro [6].
- Inhibits polyubiquitin synthesis by the ubiquitin-conjugating enzyme E2-25K [7].
- Cardioprotective [10].
- Anti-inflammatory [11].
- Shows analgesic effect by targeting TRPV1 channel activity [11].
- Neuroprotective. Inhibits NMDA receptors [12].
Product References
- The isolation and antioxidative effects of vitexin from Acer palmatum: J.H. Kim, et al.; Arch. Pharm. Res. 28, 195 (2005)
- Vitexin, an HIF-1alpha inhibitor, has anti-metastatic potential in PC12 cells: H.J. Choi, et al.; Mol. Cells 22, 291 (2006)
- Antioxidant constituents in the dayflower (Commelina communis L.) and their alpha-glucosidase-inhibitory activity: M. Shibano, et al.; J. Nat. Med. 62, 349 (2008)
- Vitexins, nature-derived lignan compounds, induce apoptosis and suppress tumor growth: Y. Zhou, et al.; Clin. Cancer Res. 15, 5161 (2009)
- Vitexin, orientin and other flavonoids from Spirodela polyrhiza inhibit adipogenesis in 3T3-L1 cells: J. Kim, et al.; Phytother. Res. 24, 1543 (2010)
- Synthesis and characterization of SIRT6 protein coated magnetic beads: identification of a novel inhibitor of SIRT6 deacetylase from medicinal plant extracts: M. Yasuda, et al.; Anal. Chem. 83, 7400 (2011)
- Vitexin inhibits polyubiquitin synthesis by the ubiquitin-conjugating enzyme E2-25K: K.M. Helms, et al.; Nat. Prod. Commun. 6, 1411 (2011)
- Vitexin and isovitexin from the Leaves of Ficus deltoidea with in-vivo α-glucosidase inhibition: C.Y. Choo, et al.; J. Ethnopharmacol. 142, 776 (2012)
- Apoptosis triggered by vitexin in U937 human leukemia cells via a mitochondrial signaling pathway: C.Y. Lee, et al.; Oncol. Rep. 28, 1883 (2012)
- Vitexin protects against cardiac hypertrophy via inhibiting calcineurin and CaMKII signaling pathways: C.C. Lu, et al.; Naunyn Schmiedebergs Arch. Pharmacol. 386, 747 (2013)
- Vitexin inhibits inflammatory pain in mice by targeting TRPV1, oxidative stress, and cytokines: S.M. Borghi, et al.; J. Nat. Prod. 76, 1141 (2013)
- The novel p53-dependent metastatic and apoptotic pathway induced by vitexin in human oral cancer OC2 cells: S.H. Yang, et al.; Phytother. Res. 27, 1154 (2013)
- Neuroprotective effects of vitexin by inhibition of NMDA receptors in primary cultures of mouse cerebral cortical neurons: L. Yang, et al.; Mol. Cell Biochem. 386, 251 (2014)
- A review on the pharmacological effects of vitexin and isovitexin: M. He, et al.; Fitoterapia 115, 74 (2016)
- Molecular targets of vitexin and isovitexin in cancer therapy: a critical review: K. Ganesan & B. Xu; Ann. N. Y. Acad. Sci. 1401, 102 (2017)
- Vitexin induces apoptosis by suppressing autophagy in multi-drug resistant colorectal cancer cells: M. Bhardwaj, et al.; Oncotarget 9, 3278 (2018)