Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
AdipoGen Life Sciences
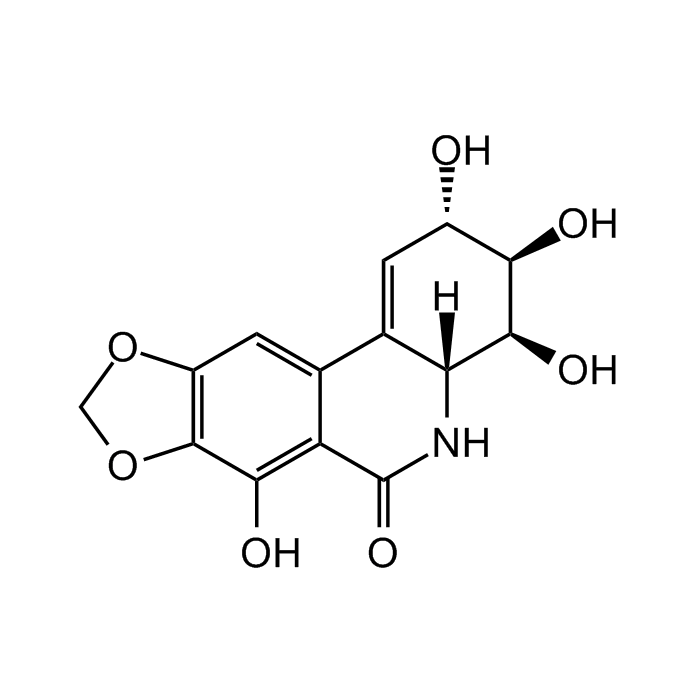
Narciclasine
As low as
90
CHF
CHF 90.00
In stock
Only %1 left
AG-CN2-0524-C500500 µgCHF 90.00
AG-CN2-0524-M0011 mgCHF 160.00
| Product Details | |
|---|---|
| Synonyms | (+)-Narciclasine; (+)-Lycoricidinol; BRN1087400; NSC266535 |
| Product Type | Chemical |
| Properties | |
| Formula |
C14H13NO7 |
| MW | 307.3 |
| CAS | 29477-83-6 |
| RTECS | JI5000000 |
| Source/Host Chemicals | Isolated from Narcissus sp. |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | Pale grey powder. |
| Solubility | Soluble in DMSO (30mg/ml). |
| Identity | Determined by 1H-NMR. |
| InChi Key | LZAZURSABQIKGB-AEKGRLRDSA-N |
| Smiles | OC1=C(C(N[C@]2([H])C3=C[C@H](O)[C@@H](O)[C@H]2O)=O)C3=CC4=C1OCO4 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice | Protect from light. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
- Plant growth modulator/inhibitor.
- Anticancer agent. Exhibits antiproliferative and pro-apoptotic effects in carcinoma cells and displays cytotoxic activity against a panel of 60 cancer cell lines. Shown to promote autophagy-induced apoptosis and to slow cell cycle progression.
- Inhibits protein synthesis by interacting with the 60S ribosome subunit.
- Regulates the Rho/ROCK/LIM kinase/cofilin pathway. Stimulates RhoA activation and induces actin polymerization.
- Anti-obesity agent. Attenuates diet-induced obesity (DIO) in mice by promoting oxidative metabolism in skeletal muscle and energy expenditure.
- Anti-inflammatory agent suppressing NF-κB-dependent gene transcription.
- Antiviral agent in vitro.
- Antifungal agent.
- Shown to inhibit amyloid plaque formation in an Alzheimer's disease model.
Product References
- Narciclasine: an antimitotic substance from Narcissus bulbs: G. Ceriotti; Nature 213, 595 (1967)
- Narciclasine: an antitumour alkaloid which blocks peptide bond formation by eukaryotic ribosomes: L. Carrasco, et al.; FEBS Lett. 52, 236 (1975)
- Antiviral (RNA) activity of selected Amaryllidaceae isoquinoline constituents and synthesis of related substances: B. Gabrielsen, et al.; J. Nat. Prod. 55, 1569 (1992)
- The Amaryllidaceae isocarbostyril narciclasine induces apoptosis by activation of the death receptor and/or mitochondrial pathways in cancer cells but not in normal fibroblasts: P. Dumont, et al.; Neoplasia 9, 766 (2007)
- Chemistry, biology and medicinal potential of Narciclasine and its congeners: A. Kornienko & A. Evidente: Chem. Rev. 108, 1982 (2008) (Review)
- Structure-activity relationship analysis of novel derivatives of narciclasine (an Amaryllidaceae isocarbostyril derivative) as potential anticancer agents: L. Ingrassia, et al.; J. Med. Chem. 52, 1100 (2009)
- Narciclasine, a plant growth modulator, activates Rho and stress fibers in glioblastoma cells: F. Lefranc, et al.; Mol. Cancer Ther. 8, 1739 (2009)
- Narciclasine as well as other Amaryllidaceae isocarbostyrils are promising GTP-ase targeting agents against brain cancers: G. Van Goietsenoven, et al.; Med. Res. Rev. 33, 439 (2013) (Review)
- Narciclasine inhibits the responses of Arabidopsis roots to auxin: Y. Hu, et al.; Planta 236, 597 (2012)
- Haemanthus coccineus extract and its main bioactive component narciclasine display profound anti-inflammatory activities in vitro and in vivo: S. Fuchs, et al.; J. Cell Mol. Med. 19, 1021 (2015)
- Effect of Lycoris chejuensis and Its Active Components on Experimental Models of Alzheimer's Disease: J. Kim, et al.; J. Agric. Food Chem. 63, 6979 (2015)
- Narciclasine - an Amaryllidaceae alkaloid with potent antitumor and anti-inflammatory properties: R. Furst, et al.; Planta Med. 82, 1389 (2016) (Review)
- Narciclasine attenuates diet-induced obesity by promoting oxidative metabolism in skeletal muscle: S.G. Julien, et al.; PLoS Biol. 15, e1002597 (2017)
- Metabolism: Narciclasine boosts energy metabolism: C. Greenhill; Nat. Rev. Endocrinol. 13, 189 (2017) (Review)
- Narciclasine induces autophagy-dependent apoptosis in triple-negative breast cancer cells by regulating the AMPK-ULK1 axis: C. Cao, et al.; Cell Prolif. 51, e12518 (2018)
- Insight to the antifungal properties of Amaryllidaceae constituents: J.J Nair & J. van Staden; Phytomedicine 73, 152753 (2020) (Review)





