Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
AdipoGen Life Sciences
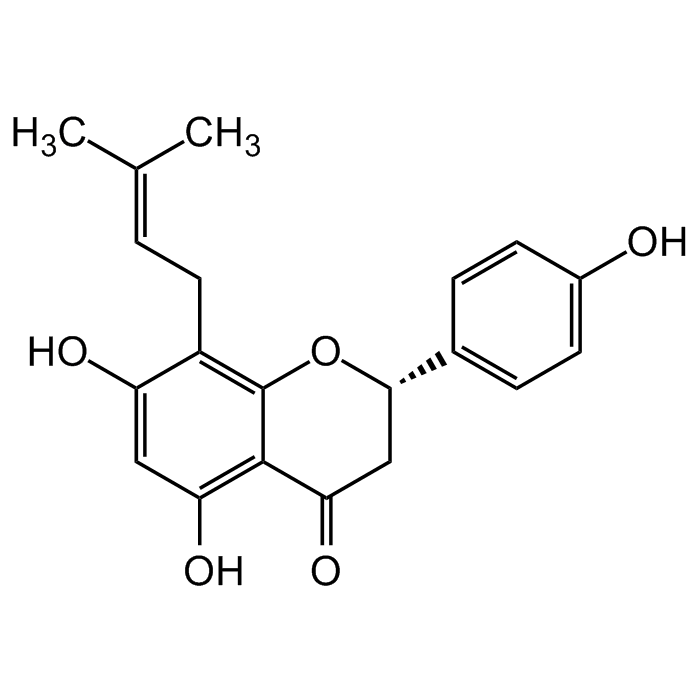
8-Prenylnaringenin
As low as
70
CHF
CHF 70.00
In stock
Only %1 left
AG-CN2-0525-M0011 mgCHF 70.00
AG-CN2-0525-M0055 mgCHF 280.00
| Product Details | |
|---|---|
| Synonyms | (-)-8-Prenylnaringenin; 8-PN; Flavaprenin; Sophoraflavanone B |
| Product Type | Chemical |
| Properties | |
| Formula |
C20H20O5 |
| MW | 340.4 |
| CAS | 53846-50-7 |
| Source/Host Chemicals | Isolated from Humulus lupulus. |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | Pale yellow powder. |
| Solubility | Soluble in DMF (10mg/ml), DMSO (5mg/ml) or EtOH (2mg/ml). |
| Identity | Determined by 1H-NMR. |
| InChi Key | LPEPZZAVFJPLNZ-SFHVURJKSA-N |
| Smiles | OC1=C(C(C[C@@H](C2=CC=C(O)C=C2)O3)=O)C3=C(C/C=C(C)/C)C(O)=C1 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice | Protect from light. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
- Prenylflavonoid non-steroidal phytoestrogen that mimicks and/or modulates endogenous estrogens via estrogen receptor binding. Inhibits both isoforms of the human estrogen receptor (ER; IC50s = 57nM and 68nM for ERα and ERβ, respectively). Acts as a full agonist of ERα.
- Cancer chemopreventive agent. Shown to decrease ROS and increase the oxidative phosphorylation system (OXPHOS).
- Anticancer agent with antiangiogenic and antiproliferative properties. Shown to inhibit cell cycle progression, induce apoptosis in MCF-7 cells and induce autophagy in prostate cancer cell lines.
- Potent aromatase inhibitor (IC50=65nM) and cellular histone deacetylases (HDACs) inhibitor.
- Anti-diabetic agent. Activates AMPK signaling consequently suppressing lipogenesis. Shown to prevent body weight gain, improve insulin resistance and glucose tolerance.
- Potent uncompetitive tight-binding inhibitor of human aldose reductase AKR1B1 (Ki=0.71μM) and of human AKR1B10 (Ki=1.95μM), both pharmacological targets in cancer therapy (AKR1B10) and in the treatment of diabetic complications (AKR1B1).
- Anti-osteoporetic agent. Effective in vivo, suppressing loss of bone mineral density.
- Anti-inflammatory and vascularprotective compound.
- Potential lead structure for potential new therapeutic agents.
Product References
- Identification of a potent phytoestrogen in hops (Humulus lupulus L.) and beer: S.R. Milligan, et al.; J. Clin. Endocrinol. Metab. 84, 2249 (1999)
- The endocrine activities of 8-prenylnaringenin and related hop (Humulus lupulus L.) flavonoids: S.R. Milligan, e al.; J. Clin. Endocrinol. Metab. 85, 4912 (2000)
- Oestrogenic activity of the hop phyto-oestrogen, 8-prenylnaringenin: S. Milligan, et al.; Reproduction 123, 235 (2002)
- 8-Prenyl naringenin is a potent ERalpha selective phytoestrogen present in hops and beer: O. Schaefer, et al.; J. Steroid Biochem. Mol. Biol. 84, 359 (2003)
- 8-prenylnaringenin, a novel phytoestrogen, inhibits angiogenesis in vitro and in vivo: M.S. Pepper, et al.; J. Cell Physiol. 199, 98 (2004)
- Effect of hop (Humulus lupulus L.) flavonoids on aromatase (estrogen synthase) activity: R. Monteiro, et al.; J. Agric. Food Chem. 54, 2938 (2006)
- Subtle side-chain modifications of the hop phytoestrogen 8-prenylnaringenin result in distinct agonist/antagonist activity profiles for estrogen receptors α and β: F. Roelens, et al.; J. Med. Chem. 49, 7357 (2006)
- Anti-proliferative properties of prenylated flavonoids from hops (Humulus lupulus L.) in human prostate cancer cell lines: L. Delmulle, et al.; Phytomedicine 13, 732 (2006)
- Modulation of breast cancer cell survival by aromatase inhibiting hop (Humulus lupulus L.) flavonoids: R. Monteiro, et al.; J. Steroid Biochem. Mol. Biol. 105, 124 (2007)
- 8-Prenylnaringenin, inhibits estrogen receptor-alpha mediated cell growth and induces apoptosis in MCF-7 breast cancer cells: E. Brunelli, et al.; J. Steroid Biochem. Mol. Biol. 107, 140 (2007)
- Treatment of PC-3 and DU145 prostate cancer cells by prenylflavonoids from hop (Humulus lupulus L.) induces a caspase-independent form of cell death: L. Delmulle, et al.; Phytother. Res. 22, 197 (2008)
- Comparison of the phytohormones genistein, resveratrol and 8-prenylnaringenin as agents for preventing osteoporosis: S. Sehmisch, et al.; Planta Med. 74, 794 (2008)
- Anti-inflammatory and vascularprotective properties of 8-prenylapigenin: T. Paoletti, et al.; Eur. J. Pharmacol. 620, 120 (2009)
- A comparison of the anticancer properties of isoxanthohumol and 8-prenylnaringenin using in vitro models of colon cancer: P. Allsopp, et al.; Biofactors 39, 441 (2013)
- The prenyl group contributes to activities of phytoestrogen 8-prenynaringenin in enhancing bone formation and inhibiting bone resorption in vitro: L.G. Ming, et al.; Endocrinology 154, 1202 (2013)
- Effect of xanthohumol and 8-prenylnaringenin on MCF-7 breast cancer cells oxidative stress and mitochondrial complexes expression: M.M. Blanquer-Rossello, et al.; J. Cell Biochem. 114, 2785 (2013)
- Xanthohumol and 8-prenylnaringenin ameliorate diabetic-related metabolic dysfunctions in mice: R. Costa, et al.; J. Nutr. Biochem. 45, 39 (2017)
- The hop-derived compounds xanthohumol, isoxanthohumol and 8-prenylnaringenin are tight-binding inhibitors of human aldo-keto reductases 1B1 and 1B10: J.M. Seliger, et al.; J. Enzyme Inhib. Med. Chem. 33, 607 (2018)
- 6- and 8-Prenylnaringenin, Novel Natural Histone Deacetylase Inhibitors Found in Hops, Exert Antitumor Activity on Melanoma Cells: S. Venturelli, et al.; Cell Physiol. Biochem. 51, 543 (2018)





