Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
AdipoGen Life Sciences
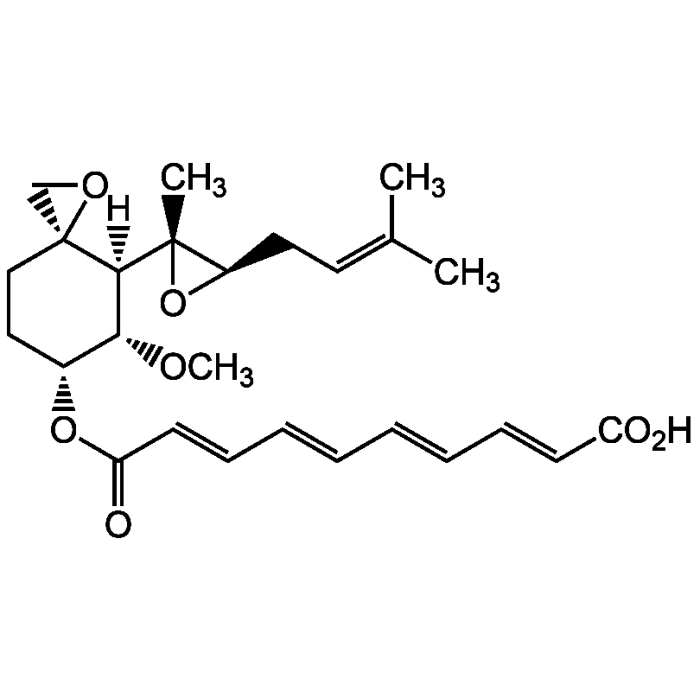
Fumagillin
As low as
60
CHF
CHF 60.00
In stock
Only %1 left
AG-CN2-0529-M0011 mgCHF 60.00
AG-CN2-0529-M0055 mgCHF 180.00
AG-CN2-0529-M02525 mgCHF 450.00
Replaces BVT-0424
| Product Details | |
|---|---|
| Synonyms | Amebacilin; Fugilin; Fumidil; Fumadil B; NSC9168; U5762 |
| Product Type | Chemical |
| Properties | |
| Formula |
C26H34O7 |
| MW | 458.6 |
| Merck Index | 14: 4286 |
| CAS | 23110-15-8 |
| RTECS | HE1750000 |
| Source/Host Chemicals | Isolated from Aspergillus fumigatus. |
| Purity Chemicals | ≥98% (1H-NMR) |
| Appearance | Off-white solid. |
| Solubility | Soluble in DMSO, acetone or chloroform. Slightly soluble in methanol (5mg/ml) or ethanol. Insoluble in water. |
| InChi Key | NGGMYCMLYOUNGM-IWMBURASSA-N |
| Smiles | [H][C@@]1([C@H](OC)[C@@H](CC[C@]11CO1)OC(=O)\C=C\C=C\C=C\C=C\C(O)=O)[C@@]1(C)O[C@@H]1CC=C(C)C |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice | Protect from light. |
| Use/Stability |
Stable for at least 2 years after receipt when stored at -20°C. Store solutions at -20°C in the dark. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
- Meroterpenoid antibiotic.
- Anticancer, antimicrobial, antimalarial and amoebicidal compound. Potent, selective and covalent inhibitor of methionine aminopeptidase-2 (MetAP2).
- Anti-angiogenic by impairing the growth of endothelial cells and altering gene expression.
- Inhibits endothelial cell proliferation in vitro and tumor-induced angiogenesis in vivo.
- Suppresses the HIV-1 infection of human macrophages through the inhibition of HIV-1 viral protein R (Vpr) activity.
- Inhibits neovascularization and might be useful in non-tumor diseases such as diabetic retinopathy, arthritis and psoriasis, which involve neovascularisation processes.
- Reduces diet-induced adipose tissue formation in mice, independent of its effects on angiogenesis.
Product References
- Antibiotic substance produced by Aspergillus fumigatus: T.E. Eble, et al.; Antibiot. Chemother. 1, 54 (1951)
- Synthetic analogues of fumagillin that inhibit angiogenesis and suppress tumour growth: D. Ingber, et al.; Nature 348, 555 (1990)
- The anti-angiogenic agent fumagillin covalently binds and inhibits the methionine aminopeptidase, MetAP-2: N. Sin, et al.; PNAS 94, 6099 (1997)
- Inhibition of Angiogenesis In Vivo by ets-1 Antisense Oligonucleotides-Inhibition of Ets-1 Transcription Factor Expression by the Antibiotic Fumagillin: N. Wernert, et al.; Angew. Chem. Int. Ed. Engl. 38, 3228 (1999)
- Structure elucidation of fumagillin-related natural products: J. Halasz, et al.; Tetrahedron 56, 10081 (2000)
- A re-evaluation of fumagillin selectivity towards endothelial cells: S. Rodriguez-Nieto, et al.; Anticancer Res. 21, 3457 (2001)
- Fumagillin treatment of intestinal microsporidiosis: J. Molina, et al.; N. Engl. J. Med. 346, 1963 (2002)
- Early genetic mechanisms underlying the inhibitory effects of endostatin and fumagillin on human endothelial cells: C.M. Mazzanti, et al.; Genome Res. 14, 1585 (2004)
- Fumagillin suppresses HIV-1 infection of macrophages through the inhibition of Vpr activity: N. Watanabe, et al.; FEBS Lett. 580, 2598 (2006)
- Fumagillin: an anti-infective as a parent molecule for novel angiogenesis inhibitors: B. Lefkove, et al.; Expert Rev. Anti-Infect. Ther. 5, 573 (2007)
- Fumagillin and fumarranol interact with P. falciparum methionine aminopeptidase 2 and inhibit malaria parasite growth in vitro and in vivo: X. Chen, et al.; Chem. Biol. 16, 193 (2009)
- Fumagillin inhibits colorectal cancer growth and metastasis in mice: in vivo and in vitro study of anti-angiogenesis: L. Hou, et al.; Pathol. Int. 59, 448 (2009)
- Syntheses of fumagillin and ovalicin: J. Yamaguchi & Y. Hayashi; Chem. Eur. J. 16, 3884 (2010)
- Fumagillin reduces adipose tissue formation in murine models of nutritionally induced obesity: H.R. Lijnen, et al.; Obesity 18, 2241 (2010)
- Inhibition of neutrophil function following exposure to the Aspergillus fumigatus toxin fumagillin: J.P. Fallon, et al.; J. Med. Microbiol. 59, 625 (2010)
- Fumagillin and structurally related molecules as source of new drugs: D. Gamba-Sanches; Mini-Rev. Org. Chem. 9, 126 (2012)
- Stimulation of suicidal erythrocyte death by fumagillin: M. Zbidah, et al.; Basic Clin. Pharmacol. Toxicol. 112, 346 (2013)





