Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
AdipoGen Life Sciences
Z-VAD-FMK (Cell permeable)

| Product Details | |
|---|---|
| Synonyms | Z-VAD(OMe)-FMK; Z-Val-Ala-DL-Asp(OMe)-FMK; pan-Caspase Inhibitor |
| Product Type | Chemical |
| Properties | |
| Formula |
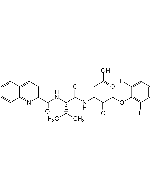
C22H30FN3O7 |
| MW | 467.5 |
| Sequence |
Z-Val-Ala-DL-Asp(OMe)-fluoromethylketone |
| CAS | 187389-52-2 |
| Purity Chemicals | ≥95% (HPLC) |
| Appearance | White solid. |
| Solubility | Soluble in DMSO, acetonitrile or dimethyl formamide. |
| Other Product Data |
Prepare a 10mM stock solution of the caspase inhibitor in high quality DMSO. Add 2μl of stock solution to 1ml of culture medium containing cells to give 20μM final concentration. Effective final concentrations are estimated to be 5-20μM. If a particular inhibitor is applied at higher concentrations, specificity for an individual caspase or even for the caspase family (>100μM) might be compromised. DMSO concentrations above 0.2% may cause cellular toxicity, thus masking the effect of the caspase inhibitor. For in vivo or in vitro experiments extending 12-48 hours, fresh inhibitor may have to be added (injected) due to inactivation of the inhibitor by endogenous cysteine proteases. |
| InChi Key | MIFGOLAMNLSLGH-SXUUOERCBK |
| Smiles | [H][C@](NC(=O)OCC1=CC=CC=C1)(C(C)C)C(=O)N[C@@H](C)C(=O)NC(CC(=O)OC)C(=O)CF |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice | Keep cool and dry. |
| Use/Stability | Stable for at least 3 years after receipt when stored at -20°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
- Cell permeable, non-selective broad-spectrum caspase inhibitor [1, 3, 7, 8]. Binds irreversibly to the catalytic site of caspase proteases [1].
- The peptide is O-methylated in the P1 position on aspartic acid, providing enhanced stability and increased cell permeability [1].
- Inhibits ICE-family protease/caspase processing, leading to apoptosis and autophagy induction [2-4, 6, 9].
- Decreases proteasome activity [5].
- Potent inhibitor of caspase-1 activation in NLRP3/NALP3-induced cells [10].
- Used in apoptosis and inflammasome studies.
- Benzyloxycarbonyl-Val-Ala-Asp (OMe) fluoromethylketone (Z-VAD.FMK) inhibits apoptosis by blocking the processing of CPP32: E.A. Slee, et al.; Biochem. J. 315, 21 (1996)
- Different interleukin-1 beta converting enzyme (ICE) family protease requirements for the apoptotic death of T lymphocytes triggered by diverse stimuli: A. Sarin, et al.; J. Exp. Med. 184, 2445 (1996)
- Processing/activation of at least four interleukin-1beta converting enzyme-like proteases occurs during the execution phase of apoptosis in human monocytic tumor cells: M. MacFarlane, et al.; J. Cell Biol. 137, 469 (1997)
- Processing/activation of caspases, -3 and -7 and -8 but not caspase-2, in the induction of apoptosis in B-chronic lymphocytic leukemia cells: D. King, et al.; Leukemia 12, 1553 (1998)
- Proteasome activities decrease during dexamethasone-induced apoptosis of thymocytes: J. Beyette, et al.; Biochem. J. 332, 315 (1998)
- JNK (c-Jun N-terminal kinase) and p38 activation in receptor-mediated and chemically-induced apoptosis of T-cells: differential requirements for caspase activation: M. MacFarlane, et al.; Biochem. J. 348, 93 (2000)
- Statin-induced proinflammatory response in mitogen-activated peripheral blood mononuclear cells through the activation of caspase-1 and IL-18 secretion in monocytes: W.R. Coward, et al.; J. Immunol. 176, 5284 (2006)
- Broad-spectrum caspase inhibitors: from myth to reality? D. Chauvier, et al.; Cell Death Differ. 14, 387 (2007)
- A calpain-like protease inhibits autophagic cell death: D.T. Madden, et al.; Autophagy 3, 519 (2007)
- Malarial hemozoin is a Nalp3 inflammasome activating danger signal; C. Dostert, et al.; PLoS One 4, e6510 (2009)
- Kinesin light chain-4 depletion induces apoptosis of radioresistant cancer cells by mitochondrial dysfunction via calcium ion influx: J.H. Baek, et al.; Cell Death Dis. 496, (2018)