Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
AdipoGen Life Sciences
Lipofermata
As low as
80
CHF
CHF 80.00
In stock
Only %1 left
AG-CR1-3542-M01010 mgCHF 80.00
AG-CR1-3542-M05050 mgCHF 240.00
| Product Details | |
|---|---|
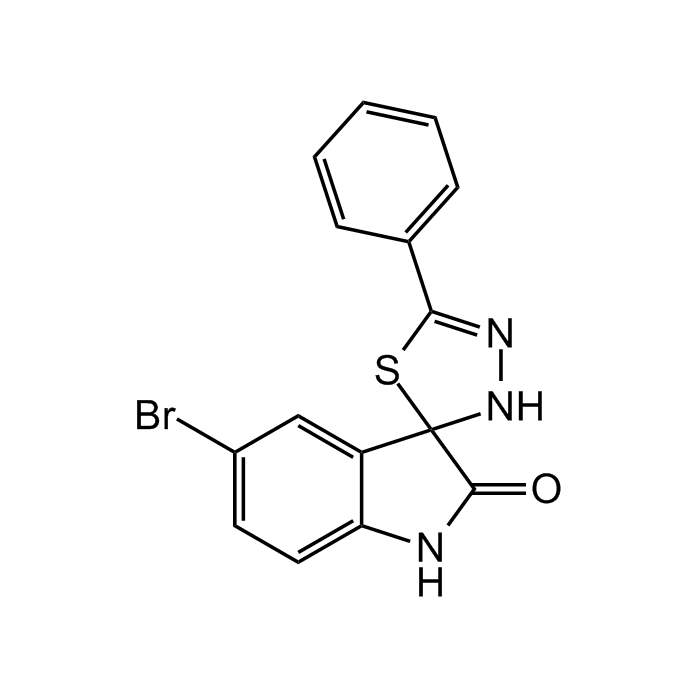
| Synonyms | 5′-Bromo-5-phenyl-spiro[3H-1,3,4-thiadiazole-2,3′-indoline]-2′-one; CB16.2 |
| Product Type | Chemical |
| Properties | |
| Formula |
C15H10BrN3OS |
| MW | 360.2 |
| CAS | 297180-15-5 |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | Off-white solid. |
| Solubility | Soluble in DMSO (20mg/ml) and DMF (25mg/ml). Slightly soluble in Ethanol (1mg/ml). |
| InChi Key | RRBYYBWDUNSVAW-UHFFFAOYSA-N |
| Smiles | BrC1=CC=C(NC(C23NN=C(C4=CC=CC=C4)S3)=O)C2=C1 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice | Keep cool and dry. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
- Lipofermata is an inhibitor of fatty acid transport, blocking fatty acid transport protein 2 (FATP2), and was identified afterwards as a FATP1 inhibitor as well. Inhibition was specific for long and very long chain fatty acids but not medium (C6-C10) fatty acids in myocytes (mmC2C12), pancreatic β cells (rnINS-1E), intestinal epithelial cells (hsCaco-2) and hepatocytes (hsHepG2), as well as primary human adipocytes. The fatty acid transport proteins (FATP), when localized to the plasma membrane or at intracellular membrane junctions with the endoplasmic reticulum, function as a gate in the regulated transport of fatty acids and thus represent a therapeutic target to delimit the acquisition of fatty acids that contribute to disease as in the case of fatty acid overload, such as obesity, Typ 2 diabetes or cancers.
- Lipofermata prevented palmitate-mediated oxidative stress, induction of BiP and CHOP and cell death in a dose-dependent manner in hsHepG2 and mINS-1E cells suggesting utility in preventing fatty acid-mediated cell death pathways and lipotoxic disease.
- FATP2 selectively allows for the accumulation of arachidonic acid, which is needed for the cells to make prostaglandin E2 (PGE2), a potent inflammatory molecule that is also implicated in the immunosuppressive activity of MDSCs in cancer. Pharmacologic blockade of FATPs with the small molecule inhibitor Lipofermata abrogates lipid transport into melanoma cells and reduces melanoma growth and invasion. In combination with checkpoint inhibitors, FATP2 inhibition blocked tumor progression in mice.
- Lipofermata also shows inhibitory activity against ADAMTS-5 (Aggrecanase-2) with potential use in osteoarthritis.
Product References
- 5'-Phenyl-3'H-spiro[indoline-3,2'-[1,3,4]thiadiazol]-2-one inhibitors of ADAMTS-5 (Aggrecanase-2): M.G. Bursavich, et al.; Bioorg. Med. Chem. Lett. 17, 5630 (2007)
- Identification and characterization of small compound inhibitors of human FATP2: A. Sandoval, et al.; Biochem. Pharmacol. 79, 990 (2010)
- Chemical inhibition of fatty acid absorption and cellular uptake limits lipotoxic cell death: C. Ahowesso, et al.; Biochem. Pharmacol. 98, 167 (2015)
- Fatty Acid Transport Proteins: Targeting FATP2 as a Gatekeeper Involved in the Transport of Exogenous Fatty Acids: P.N. Black, et al.; Medchemcomm. 7, 612 (2016)
- Adipocyte-derived lipids mediate melanoma progression via FATP proteins; M. Zhang, et al.; Cancer Discov. 8, 1006 (2018)
- Fatty acid transport protein 2 reprograms neutrophils in cancer: F. Veglia, et al.; Nature 569, 73 (2019)
- Deletion of fatty acid transport protein 2 (FATP2) in the mouse liver changes the metabolic landscape by increasing the expression of PPARα-regulated genes: V.M. Perez, et al.; Genomics Proteomics 295, P5737 (2020)
- Regulation of ROS in myeloid-derived suppressor cells through targeting fatty acid transport protein 2 enhanced anti-PD-L1 tumor immunotherapy: A.O. Adeshakin, et al.; Cell Immunol. 362, 104286 (2021)
- Multiple myeloma cells induce lipolysis in adipocytes and uptake fatty acids through fatty acid transporter proteins: C. Panaroni, et al.; Blood (Epub ahead of print) (2021)