Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
AdipoGen Life Sciences
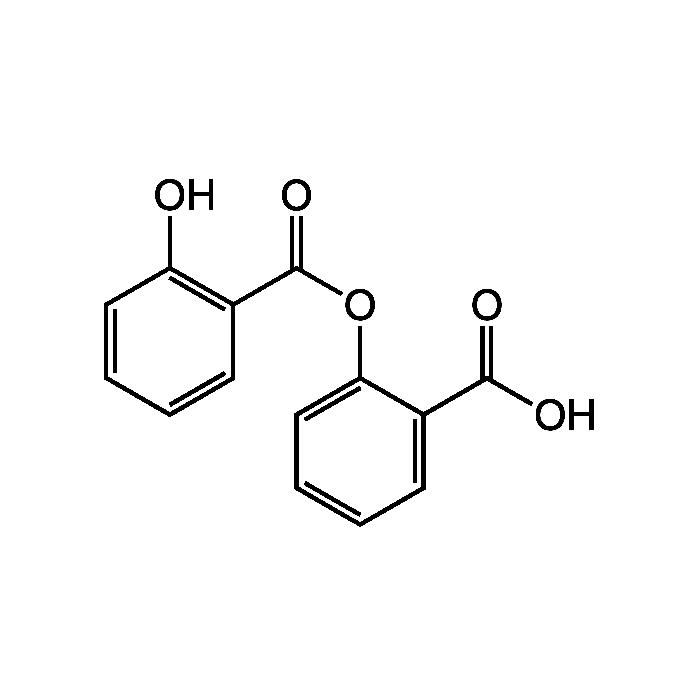
Salsalate
As low as
40
CHF
CHF 40.00
In stock
Only %1 left
AG-CR1-3574-G0011 gCHF 40.00
AG-CR1-3574-G0055 gCHF 110.00
| Product Details | |
|---|---|
| Synonyms | Salicylsalicylic acid; NSC 49171; BRN 2590908; 2-Hydroxy-2-carboxyphenyl ester-benzoic acid |
| Product Type | Chemical |
| Properties | |
| Formula |
C14H10O5 |
| MW | 258.2 |
| Merck Index | 14: 8339 |
| CAS | 552-94-3 |
| RTECS | DH2080000 |
| Purity Chemicals | ≥98% |
| Appearance | White to off-white powder. |
| Solubility | Soluble in DMSO, ethanol or dimethylformamide. Sparingly soluble in water. |
| InChi Key | WVYADZUPLLSGPU-UHFFFAOYAY |
| Smiles | OC(=O)C1=CC=CC=C1OC(=O)C1=C(O)C=CC=C1 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice | Keep cool and dry. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
- Nonacetylated salicylate.
- Non-steroidal anti-inflammatory drug (NSAID).
- Reduces pain and inflammation caused by conditions such as rheumatoid arthritis, osteoarthritis and related rheumatic conditions.
- Prostaglandin synthesis inhibitor in vivo.
- Inactivates cyclooxygenase-1 (COX-1) and -2 (COX-2).
- IKKβ/NF-κB inhibitor (at significantly higher concentrations than required for COX inhibition).
- Used to target inflammation in the treatment of insulin resistance, type 2 diabetes, or rheumatic pain.
- It reduces blood glucose concentrations in patients with type 2 diabetes, as well as in insulin-resistant patients without diabetes.
- Reduces blood glucose, triglyceride, free fatty acid and C-reactive protein concentrations, improves glucose utilization and increases circulating insulin and adiponectin concentrations in obese adults at risk for the development of type 2 diabetes as well as for patients with type 2 diabetes.
- Dimeric prodrug comprising two esterified salicylate moieties. It is advantageous over sodium salicylate because it is insoluble at the acid pH of the stomach and passes suspended but undissolved into the small intestine, sparing the gastric mucosa direct contact.
Product References
- Reduced risk of NSAID gastropathy (GI mucosal toxicity) with nonacetylated salicylate (salsalate): an endoscopic study: S. Roth, et al.; Semin. Arth. Rheum. 19, 11 (1990)
- Inhibition of NF-kappa B by sodium salicylate and aspirin: E. Kopp & S. Ghosh; Science 265, 956 (1994)
- Salicylates inhibit I kappa B-alpha phosphorylation, endothelial-leukocyte adhesion molecule expression, and neutrophil transmigration: J.W. Pierce, et al.; J. Immunol. 156, 3961 (1996)
- The anti-inflammatory agents aspirin and salicylate inhibit the activity of I(kappa)B kinase-beta: M.J. Yin, et al.; Nature 396, 77 (1998)
- Salsalate improves glycemia and inflammatory parameters in obese young adults: A. Fleischman, et al.; Diabetes Care 31, 289 (2008)
- Use of salsalate to target inflammation in the treatment of insulin resistance and type 2 diabetes: A.B. Goldfine, et al.; Clin. Transl. Sci. 1, 36 (2008)
- The effect of salsalate on insulin action and glucose tolerance in obese non-diabetic patients: results of a randomised double-blind placebo-controlled study: J. Koska, et al.; Diabetologia 52, 385 (2009)
- Nuclear factor-kappaB activation contributes to vascular endothelial dysfunction via oxidative stress in overweight/obese middle-aged and older humans: G.L. Pierce, et al.; Circulation 119, 1284 (2009)
- The effects of salsalate on glycemic control in patients with type 2 diabetes: A randomized trial: A.B. Goldfine, et al.; Ann. Intern. Med. 152, 346 (2010)
- Potential role of salicylates in type 2 diabetes: M.M. Rumore & K.S. Kim; Ann. Pharmacother. 44, 1207 (2010) (Review)
- Effects of salsalate therapy on recovery from vascular injury in female Zucker fatty rats: S.N. Murthy, et al.; Diabetes 59, 3240 (2010)
- Stimulation of human whole-body energy expenditure by salsalate is fueled by higher lipid oxidation under fasting conditions and by higher oxidative glucose disposal under insulin-stimulated conditions: R.C. Meex, et al.; J. Clin. Endocrinol. Metab. 96, 1415 (2011)
- Salsalate attenuates free fatty acid-induced microvascular and metabolic insulin resistance in humans: W. Chai, et al.; Diabetes Care 34, 1634 (2011)
- Modeling diabetes disease progression and salsalate intervention in Goto-Kakizaki rats: Y. Cao, et al.; J. Pharmacol. Exp. Ther. 339, 896 (2011)
- Salsalate improves glycemic control in patients with newly diagnosed type 2 diabetes: E. Faghihimani, et al.; Acta Diabetol. 50, 537 (2013)
- Salicylate downregulates 11β-HSD1 expression in adipose tissue in obese mice and in humans, mediating insulin sensitization: M. Nixon, et al.; Diabetes 61, 790 (2012)
- The ancient drug salicylate directly activates AMP-activated protein kinase: S.A. Hawley, et al.; Science 336, 918 (2012)
- Reduction of insulin resistance and plasma glucose level by salsalate treatment in persons with prediabetes: E. Faghihimani, et al.; Endocr. Pract. 18, 826 (2012)
- Reducing acetylated tau is neuroprotective in brain injury: MK. Shin, et al.; Cell 184, 2715 (2021)