Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
AdipoGen Life Sciences
PTACH [NCH 51]
As low as
90
CHF
CHF 90.00
In stock
Only %1 left
AG-CR1-3667-M0055 mgCHF 90.00
AG-CR1-3667-M02525 mgCHF 320.00
| Product Details | |
|---|---|
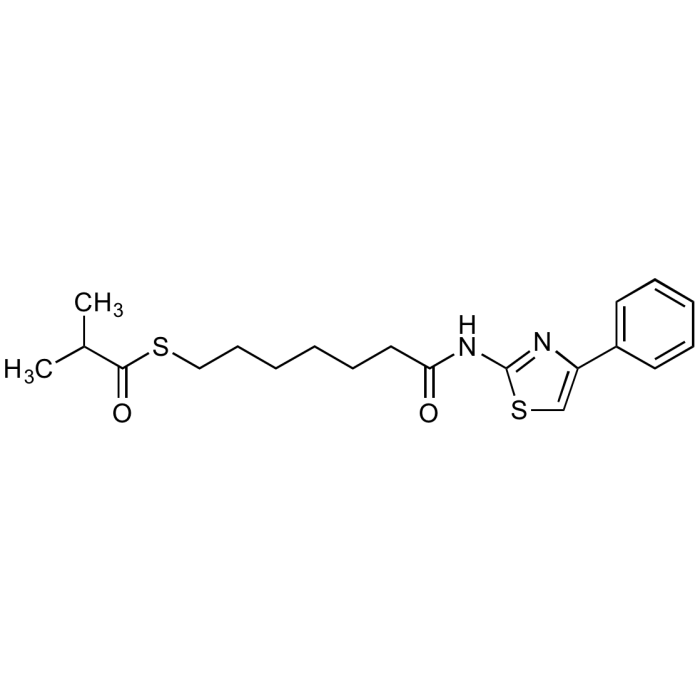
| Synonyms | Cpd 51; S-(7-Oxo-7-((4-phenylthiazol-2-yl)amino)heptyl) 2-methylpropanethioate |
| Product Type | Chemical |
| Properties | |
| Formula |
C20H26N2O2S2 |
| MW | 390.56 |
| CAS | 848354-66-5 |
| Purity Chemicals | ≥97% (HPLC) |
| Appearance | White crystals. |
| Solubility | Soluble in DMSO (25mg/ml) or ethanol (10mg/ml). Insoluble in water. |
| Identity | Determined by 1H-NMR. |
| InChi Key | MDYDGUOQFUQOGE-UHFFFAOYSA-N |
| Smiles | CC(C)C(=O)SCCCCCCC(=O)NC1=NC(=CS1)C1=CC=CC=C1 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice |
Keep cool and dry. Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
- Potent non-hydroxamate HDAC inhibitor (HDACi) (IC50: 32, 48 and 41nM for HDAC4, HDAC1 and HDAC6, respectively).
- Cell permeable prodrug that is intracellularly converted to the potent HDAC inhibitor NCH 31.
- Predicted to exhibit a similar HDAC binding mode as that of SAHA, interacting with the active-site zinc targeting group.
- Shown to exhibit comparable antiproliferative and apoptotic activity as SAHA against various cancer cell lines. Inhibits growth of various cancer cells in vitro (EC50=1.1 - 9.1μM).
- Reactivates latent HIV-1 gene expression.
- Active against selected neurodevelopmental disorders.
- Normalizes neuronal impairment and neurodegeneration caused by ATP6V1A deficit.
Product References
- Novel inhibitors of human histone deacetylases: design, synthesis, enzyme inhibition, and cancer cell growth inhibition of SAHA-based non-hydroxamates: T. Suzuki, et al.; J. Med. Chem. 48, 1019 (2005)
- Proteome analyses of the growth inhibitory effects of NCH-51, a novel histone deacetylase inhibitor, on lymphoid malignant cells: T. Sanda, et al.; Leukemia 21, 2344 (2007)
- Identification of a potent and stable antiproliferative agent by the prodrug formation of a thiolate histone deacetylase inhibitor: T. Suzuki, et al.; Bioorg. Med. Chem. Lett. 17, 1558 (2007)
- Novel histone deacetylase inhibitor NCH-51 activates latent HIV-1 gene expression: A.F. Victoriano, et al.; FEBS Lett. 585, 1103 (2011)
- Immunological and pharmacological strategies to reactivate HIV-1 from latently infected cells: a possibility for HIV-1 paediatric patients? M. Martinez-Bonet, et al.; J. Virus Erad. 1, 148 (2015)
- Altered neuronal network and rescue in a human MECP2 duplication model: S. Nageshappa, et al.; Mol. Psychiatry 21, 178 (2016)
- Transformative Network Modeling of Multi-omics Data Reveals Detailed Circuits, Key Regulators, and Potential Therapeutics for Alzheimer’s Disease: M. Wang, et al.; Neuron 109, 257 (2021)