Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
BioViotica
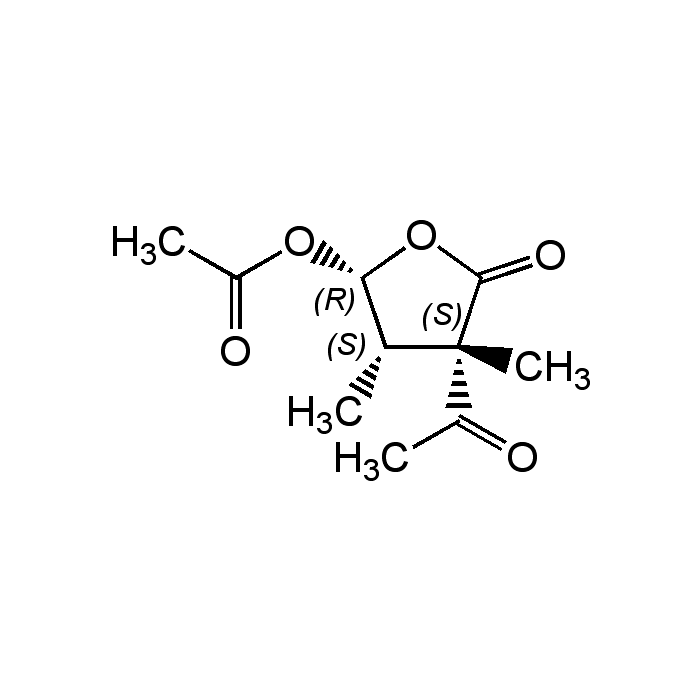
Acetomycin
As low as
115
CHF
CHF 115.00
In stock
Only %1 left
BVT-0150-M0011 mgCHF 115.00
BVT-0150-M0055 mgCHF 385.00
| Product Details | |
|---|---|
| Synonyms | NSC350598; 3-Acetyl-5-(acetyloxy)dihydro-3,4-dimethyl-2(3H)furanone; (2R,3S,4S)-4-Acetyl-3,4-dimethyl-5-oxotetrahydro-furan-2-yl acetate |
| Product Type | Chemical |
| Properties | |
| Formula |
C10H14O5 |
| MW | 214.2 |
| CAS | 510-18-9 |
| Source/Host Chemicals | Isolated from Streptomyces ramulosus. |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | White to off-white crystalline solid. |
| Solubility | Soluble in DMSO or methanol. |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by BioViotica. |
| InChi Key | OYMZTORLGBISLR-RHFNHBFPSA-N |
| Smiles | C[C@@H]1[C@H](OC(C)=O)OC(=O)[C@]1(C)C(C)=O |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Use/Stability |
Stable for at least 1 year after receipt when stored at -20°C. After reconstitution protect from light at -20°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
- Antibiotic.
- Antibacterial, antifungal and antiprotozoal.
- Cytotoxic against several tumor cell lines in vitro.
Product References
- The structure of acetomycin. Spectroscopic characterization and X-ray analysis of a bromo derivative: H. Uhr, et al.; J. Antibiot. 38, 1684 (1985)
- Biological effects of acetomycin. I. Activity against tumor cells in vitro and in vivo: S.W. Mamber, et al.; J. Antibiot. 40, 73 (1987)
- Biological effects of acetomycin. II. Inactivation by esterases in vitro: S.W. Mamber, et al.; J. Antibiot. 40, 77 (1987)
- The structure and absolute configuration of acetomycin: F.H. Cano, et al.; Acta Cryst. C. 44, 919 (1988).
- Synthesis and in vitro cytotoxicity of acetomycin and related analogs: D. Chen, et al.; Bioorg. Med. Chem. Lett. 5, 759 (1995)
- Total synthesis of (±)-acetomycin and design of esterase-resistant analogs: J. Uenishi, et al.; Chem. Pharm. Bull. 47, 517 (1999)
- Formation of chiral quaternary carbon stereocenters using silylene transfer reactions: Enantioselective synthesis of (+)-5-epi-acetomycin: S.A. Calad, et al.; Org. Lett. 9, 1037 (2007)