Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
BioViotica
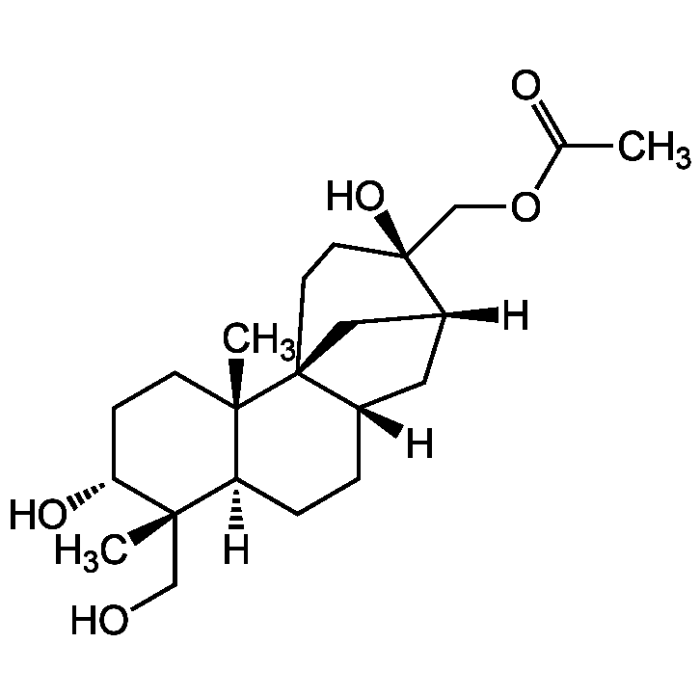
Aphidicolin 17-acetate
As low as
110
CHF
CHF 110.00
In stock
Only %1 left
BVT-0337-C250250 µgCHF 110.00
| Product Details | |
|---|---|
| Synonyms | 17-O-Acetylaphidicolin; Aphidicolin 17-monoacetate |
| Product Type | Chemical |
| Properties | |
| Formula |
C22H36O5 |
| MW | 380.5 |
| CAS | 51103-57-2 |
| Source/Host Chemicals | Isolated from Phoma sp. BS 7210. |
| Purity Chemicals | ≥96% (HPLC, 1H-NMR) |
| Appearance | White to off-white solid. |
| Solubility | Soluble in DMSO or methanol. |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by BioViotica. |
| InChi Key | GAPINCSXTLCIPV-BORIEPGUSA-N |
| Smiles | [H][C@@]12C[C@]3([H])CC[C@@]4([H])[C@](C)(CO)[C@H](O)CC[C@]4(C)[C@]3(C1)CC[C@]2(O)COC(C)=O |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice | Keep cool and dry. |
| Use/Stability |
Stable for at least 1 year after receipt when stored at -20°C. After reconstitution protect from light at -20°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
- Phytotoxin (inhibits root growth of seeding).
- Specific inhibitor of eukaryotic DNA polymerase alpha and DNA synthesis (competition with dCTP).
Product References
- Specific inhibitors of eukaryotic DNA synthesis and DNA polymerase alpha, 3-deoxyaphidicolin and aphidicolin-17-monoacetate: T. Haraguchi, et al.; Nucleic Acids Res. 11, 1197 (1983)
- 3-Deoxyaphidicolin and aphidicolin analogues as phytotoxin from Phoma betae: A. Ichihara, et al.; Agric. Biol. Chem. 48, 1687 (1984)
- Chemical modification of aphidicolin and the inhibitory effects of its derivatives on DNA polymerase alpha in vitro: S. Hiranuma, et al.; Chem. Pharm. Bull. 35, 1641 (1987)
- Inhibition of DNA polymerase alpha by aphidicolin derivatives: L. Arabshahi, et al.; Nucleic Acids Res. 16, 5107 (1988)
- Aphidicolin synthetic studies: a stereocontrolled end game: C. Rizzo, et al.; J. Chem. Soc. 5, 969 (1991)
- Metabolites from Phoma sp. 7210, associated with Aizoon canariense: J. Dai, et al.; Nat. Prod. Comm. 5, 1175 (2010)