Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
BioViotica
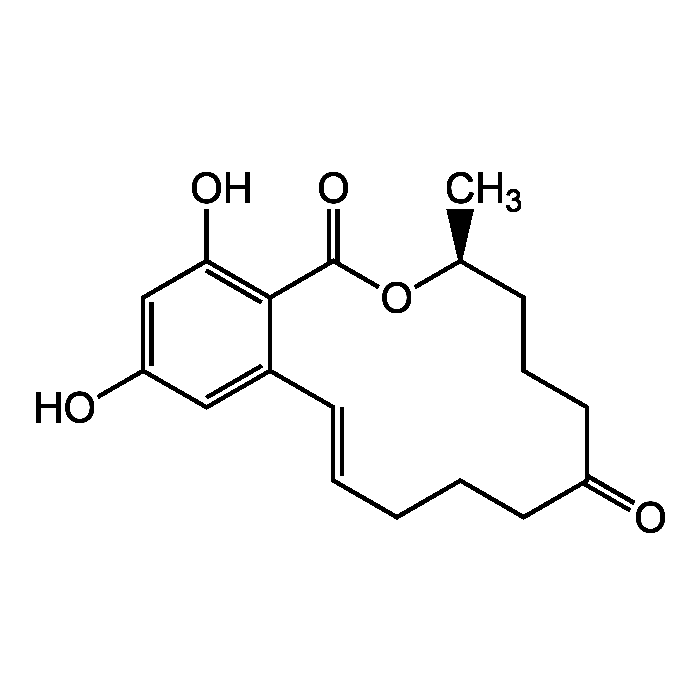
Zearalenone
As low as
75
CHF
CHF 75.00
In stock
Only %1 left
BVT-0394-M0055 mgCHF 75.00
BVT-0394-M02525 mgCHF 240.00
| Product Details | |
|---|---|
| Synonyms | Toxin F2; BRN 1350216; FES; NCI-C50226; Zenone |
| Product Type | Chemical |
| Properties | |
| Formula |
C18H22O5 |
| MW | 318.4 |
| Merck Index | 13: 10169 |
| CAS | 17924-92-4 |
| Source/Host Chemicals | Isolated from Fusarium graminearum. |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | Off-white solid. |
| Solubility | Soluble in DMSO, methanol or acetone. |
| Identity | Determined by 1H-NMR and UV. |
| Declaration | Manufactured by BioViotica. |
| InChi Key | MBMQEIFVQACCCH-QBODLPLBSA-N |
| Smiles | C[C@H]1CCCC(=O)CCC\C=C\C2=CC(O)=CC(O)=C2C(=O)O1 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice | Keep cool and dry. |
| Use/Stability |
Stable for at least 1 year after receipt when stored at -20°C. After reconstitution protect from light at -20°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
- Resorcylic acid lactone.
- Mycotoxin.
- Regulator of fungal morphogenesis.
- Mycoestrogen with anabolic and uterotrophic activity.
- Ecdysteroid antagonist.
- Gonadotropin inhibitor.
- Interleukin antagonist.
- Apoptosis inducer.
- Analytical standard in food industry.
Product References
- The structure of zearalenone: W. H. Urry, et al.; Tetrahedron Lett. 1966, 3109 (1966)
- Physicochemical data for some selected mycotoxins: A E. Pohland, et al.; Pure Appl. Chem. 54, 2219 (1982)
- Zearalenone: K. Panneerselvam, et al.; Acta Cryst. C52, 3095 (1996)
- The mycoestrogen zearalenone induces CYP3A through activation of the pregnane X receptor: X. Ding, et al.; Toxicol. Sci. 91, 448 (2006)
- Trace mycotoxin analysis in complex biological and food matrices by liquid chromatography-atmospheric pressure ionisation mass spectrometry: P. Zollner & B. Mayer-Helm; J. Chromatogr. A 1136, 123 (2006)
- The PKS4 gene of Fusarium graminearum is essential for zearalenone production: E. Lysoe, et al; Appl. Environ. Microbiol. 72, 3924 (2006)
- Chemistry and biology of resorcyclic acid lactones: N. Winssinger & S. Barluenga; Chem. Commun. 2007, 22 (2007)
- Review on the toxicity, occurrence, metabolism, detoxification, regulations and intake of zearalenone: an oestrogenic mycotoxin: A. Zinedine, et al.; Food Chem. Toxicol. 45, 1 (2007)
- Biomimetic synthesis of resorcylate natural products utilizing late stage aromatization: concise total syntheses of the marine antifungal agents 15G256ι and 15G256β: I. Navarro, et al.; JACS 130, 10293 (2008)
- Effects of exposure to zearalenone on porcine oocytes and sperm during maturation and fertilization in vitro: R. Sambuu, et al.; J. Reprod. Dev. 57, 547 (2011)
- Fusarium graminearum mycotoxins and their biosynthetic genes: H. Son & Y.-W. Lee; Mycotoxins 62, 29 (2012)
- Zearalenone induces apoptosis and necrosis in porcine granulose cells via a caspase-3- and caspase-9-dependent mitochondrial signaling pathway: L. Zhu, et al.; J. Cell. Physiol. 227, 1814 (2012)