Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
BioViotica
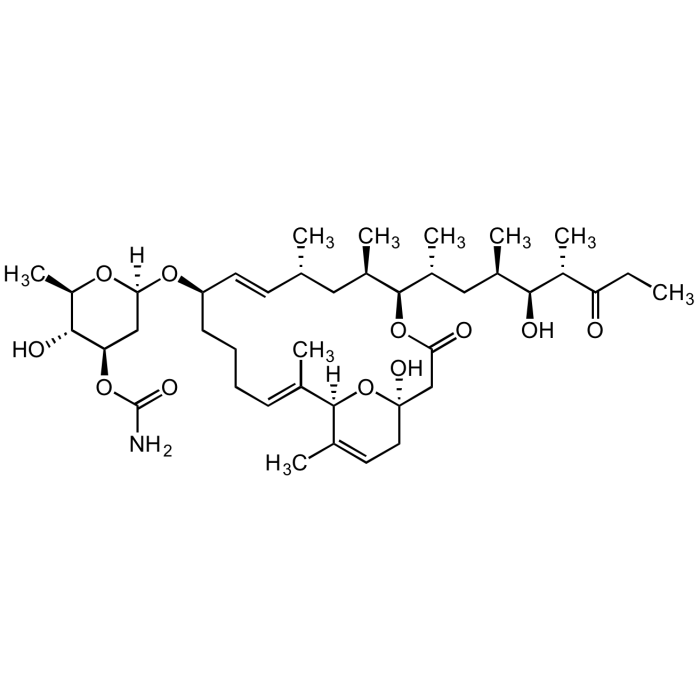
Venturicidin A
As low as
145
CHF
CHF 145.00
In stock
Only %1 left
BVT-0454-M0011 mgCHF 145.00
| Product Details | |
|---|---|
| Synonyms | Aabomycin A1 |
| Product Type | Chemical |
| Properties | |
| Formula |
C41H67NO11 |
| MW | 750.0 |
| Merck Index | 14: 9947 |
| CAS | 33538-71-5 |
| RTECS | YX4556000 |
| Source/Host Chemicals | Isolated from Streptomyces aureofaciens. |
| Purity Chemicals | ≥98% (1H-NMR, HPLC) |
| Appearance | White solid. |
| Solubility | Soluble in DMSO, methanol, acetone or dichloromethane. Insoluble in hexane or water. |
| Identity | Determined by 1H-NMR, 13C-NMR and MS. |
| Declaration | Manufactured by BioViotica. |
| InChi Key | HHQKNFDAEDTRJK-LIOWZGMGSA-N |
| Smiles | [H][C@@]1(C[C@@H](OC(N)=O)[C@H](O)[C@@H](C)O1)O[C@@H]1CCC\C=C(C)\[C@@]2([H])O[C@](O)(CC=C2C)CC(=O)O[C@H]([C@H](C)C[C@@H](C)[C@H](O)[C@H](C)C(=O)CC)[C@H](C)C[C@@H](C)\C=C\1 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | +4°C |
| Handling Advice | Protect from light when in solution. |
| Use/Stability | Stable for at least 1 year after receipt when stored at +4°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
- 20-Membered macrolide glycoside.
- Antibiotic and antifungal agent.
- Shows antitrypanosomal and antimalarial activity.
- Potent inhibitor of mitochondrial and bacterial ATP synthase.
- Inhibitor of F1F0-ATPase.
- Na+-translocating ATP synthases inhibitor.
Product References
- Venturicidin: a new antifungal antibiotic of potential use in agriculture: A. Rhodes, et al.; Nature 192, 952 (1961)
- Metabolic products of microorganismus. 102. The structure of venturicidin A and B: M. Brufani, et al.; Helv. Chim. Acta 55, 2329 (1972)
- The chemotherapy of rodent malaria, XXXI. The effect of some metabolic inhibitors upen chloroquine-induced pigment clumping (CIPC) in Plasmodium berghei: D. C. Warhurst, et al.; Ann. Trop. Med. Parasitol. 72, 203 (1978)
- Effects of inhibitors on mitochondrial adenosine triphosphatase of T. pyriformis ST.: M. D. Unitt, et al.; J. Gen. Microbiol. 126, 261 (1981)
- Inhibition of Escherichia coli H+-ATPase by venturicidin, oligomycin and ossamycin: D. S. Perlin, et al.; Biochim. Biophys. Acta 807, 238 (1985)
- Identity of aabomycin A with venturicidins: H. Akita, et al.; Agric. Biol. Chem. 54, 2465 (1990)
- An attempt to discriminate catalytic and regulatory proton binding sites in membrane-bound, thiol-reduced chloroplast ATP: M. Valerio, et al.; Biochemistry 31, 4239 (1992)
- Studies on the mechanism of oxidative phosphorylation. ATP synthesis by submitochondrial particles inhibited at F0 by venturicidin and organotin compounds: A. Matsuno-Yagi & Y. Hatefi; J. Biol. Chem. 268, 6168 (1993)
- The prokaryotic thermophilic TF1-ATPase is functionally compatible with the eukaryotic CF0-part of the chloroplast ATP sythase: J. M. Galmiche, et al.; FEBS Lett. 338, 152 (1994)
- Modification of sulfhydryl groups in the g-subunit of chloroplast-coupling factor 1 affects the proton slip through the ATP synthase: Y. Evron, et al.; Plant Physiol. 115, 1549 (1997)
- Selective and potent in vitro antitrypanosomal activities of ten microbial metabolites: K. Otoguro, et al.; J. Antibiot. 61, 372 (2008)
- A novel 11-kDa inhibitory subunit in the F1FO ATP synthase of Paracoccus denitrificans and related alpha-proteobacteria: E. Morales-Rios, et al.; FASEB J. 24, 599 (2010)
- Venturicidin C, a new 20-membered macrolide produced by Streptomyces sp. TS-2-2: K. Shaaban et al.; J. Antibiot. 67, 223 (2014)