Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
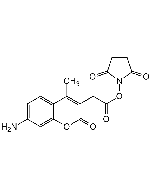
AMCA-H
| Product Details | |
|---|---|
| Synonyms | 7-Amino-4-methyl-3-coumarinylacetic acid |
| Product Type | Chemical |
| Properties | |
| Formula |
C12H11NO4 |
| MW | 233.22 |
| CAS | 106562-32-7 |
| Source/Host Chemicals | Synthetic. |
| Purity Chemicals | ≥90% (HPLC) |
| Appearance | Brown solid. |
| Solubility | Soluble in DMSO or DMF. |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | QEQDLKUMPUDNPG-UHFFFAOYSA-N |
| Smiles | CC1=C(CC(O)=O)C(=O)OC2=C1C=CC(N)=C2 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
AMCA is one of the brightest amine-reactive blue fluorescent dyes useful for immunofluorescence and fluorescent labeling (Ex/Em: 353/455nm). It is quite photostable, and its fluorescence is pH-independent from pH 4 to 10. The properties include a relatively large Stoke's shift and resistance to photobleaching. Reactive AMCA derivatives are used as contrasting probes for double and triple labeling in immunofluorescence microscopy, arrays and in situ hybridization. NHS-AMCA and Sulfo-NHS-AMCA are reactive towards primary amine groups on antibodies, proteins, peptides and other biomolecules. AMCA-Hydrazide is used to label glycosylation sites or for conjugation to carboxyl groups using the crosslinker EDC.
(1) G-L. Ferri et al.; J. Histochem. Cytochem. 45(2), 155 (1997) | (2) B. Ufhake et al.; J. Neurosc. Meth. 40(1), 39 (1991) | (3) M. W. Wessendorf et al.; J. Histochem. Cytochem. 38(1), 87 (1990)