Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
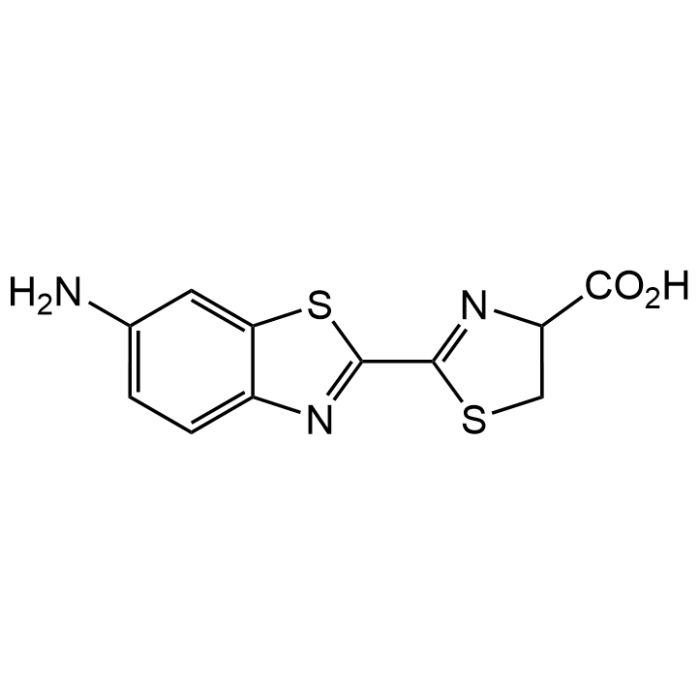
6-Amino-DL-luciferin
As low as
84
CHF
CHF 84.00
In stock
Only %1 left
CDX-A0094-M0011 mgCHF 84.00
CDX-A0094-M0055 mgCHF 168.00
| Product Details | |
|---|---|
| Synonyms | 4,5-Dihydro-2[6-amino-2-benzthiazolyl]-4-thiazole carboxylic acid |
| Product Type | Chemical |
| Properties | |
| Formula | C11H9N3O2S2 |
| MW | 279.34 |
| CAS | 118969-27-0 |
| Source/Host Chemicals | Synthetic |
| Purity Chemicals | ≥95% (HPLC) |
| Appearance | Solid. |
| Solubility | Soluble in water. |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | HKSJKXOOBAVPKR-UHFFFAOYSA-N |
| Smiles | NC1=CC=C(N=C(C2=NC(C(O)=O)CS2)S3)C3=C1 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +20°C |
| Long Term Storage | +4°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at +4°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
This is the racemic mixture containing both the D- and L-enantiomers of 6-amino-luciferin. While the D-enantiomer is active with firefly luciferase, the L-enantiome is less active. This amino analog of the common D-luciferin substrate is used in measuring firefly luciferase activity. The cell permeable reagent can be used when considering design of proluminescent bioconjugates (peptidase substrates) of luciferin for ultrasensitive luminescent assays. In bioluminescent protease assays peptides or amino acids are conjugated to the luciferin analog 6-amino-D-luciferin. These substrates are useful for ultrasensitive analysis of a variety of protease enzymes including various caspases and cathepsins, chymotrypsin, trypsin, elastase, kallikrein, thrombin, ficin, bromoalain, plasmin, papain, ficin and many others or even for use in vivo tracing of luciferase transfected cells in living tissues. The resulting bioluminescence spectrum of reaction of 6-Amino-D-luciferin is very similar to that observed for the natural substrate D-luciferin. Peptidase assays using a bioluminogenic substrate can be easily adapted to 96-well formats for HTS-based assays. Using this principle, it is possible to design an assay to detect almost any peptidase or protease activity via bioluminescence by using a substrate with a suitable peptide sequence. Applications in pathogen detection, discovery of protease inhibitors, probing cell physiology and assessing protease activity in oncogenesis are possible at extraordinary sensitivity.
Product References
(1) E.H. White, et al.; JACS 88, 2015 (1966) | (2) S. Schramm, et al.; Angew. Int. Ed. Chem. 57, 9538 (2018)





