Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
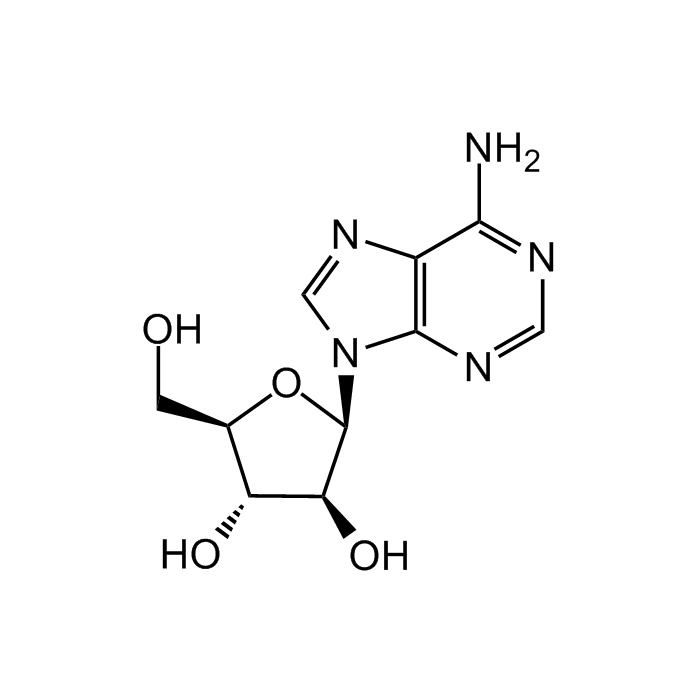
Adenine 9-β-D-arabinofuranoside
| Product Details | |
|---|---|
| Synonyms | Adenine Arabinoside; Arabinosyladenine; 9-β-D-Arabinofuranosyladenine; Ara-A; Vidarabine; NSC 247519; NSC 404241; Vira-A |
| Product Type | Chemical |
| Properties | |
| Formula |
C10H13N5O4 |
| MW | 267.24 |
| CAS | 5536-17-4 |
| RTECS | AU6200000 |
| Purity Chemicals | ≥99% (HPLC) |
| Appearance | White to off-white powder. |
| Solubility | Soluble in DMSO (20mg/ml) or DMF (5mg/ml). Slightly soluble in water (1mg/ml). |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | OIRDTQYFTABQOQ-UHTZMRCNSA-N |
| Smiles | NC1=NC=NC2=C1N=CN2[C@@H]3O[C@H](CO)[C@@H](O)[C@@H]3O |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | -20°C |
| Long Term Storage | -20°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Adenine 9-β-D-arabinofuranoside is an analog of the nucleoside adenosine that has antiviral properties. It acts as a prodrug that, once phosphorylated by cellular enzymes, acts as both substrate and inhibitor of DNA polymerase. It has been shown to be effective against H. simplex, V. zoster and Epstein-Barr viruses. It is a potent inhibitor of AMP-activated protein kinase (AMPK) in liver, muscle and cardiac cells H9c2. It is also a neurotransmitter that acts as the preferred endogenous agonist at all adenosine receptor subtypes and showed anti-neoplastic activities. It has been shown to have inhibitory activity against SARS-CoV-2-ACE2 binding.
(1) S. Yoshida, et al.; J. Biochem. 98, 433 (1985) | (2) W.B. Parker & Y. Cheng; Mol. Pharmacol. 31, 146 (1986) | (3) B. Bean; Clin. Microbiol. Rev. 5, 146 (1992) | (4) Y. Honma & N. Nitsu; Leuk. Lymphoma 399, 57 (2000) | (5) H. Kimura, et al.; J. Pediatr. Hematol. Oncol. 23, 294 (2001) | (6) A. Kishimoto, et al.; Mol. Biotechnol. 32, 17 (2006) | (7) M. Suzuki, et al.; Antiviral Res. 72, 157 (2006) | (8) K.A. Jacobson & Z.-G. Gao; Nat. Rev. Drug Discov. 5, 247 (2006) (Review) | (9) S. Holzer, et al.; ACS Chem. Biol. 14, 1904 (2019) | (10) M. Prajapat, et al.; J. Mol. Graph. Model 101, 107716 (2020)





