Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
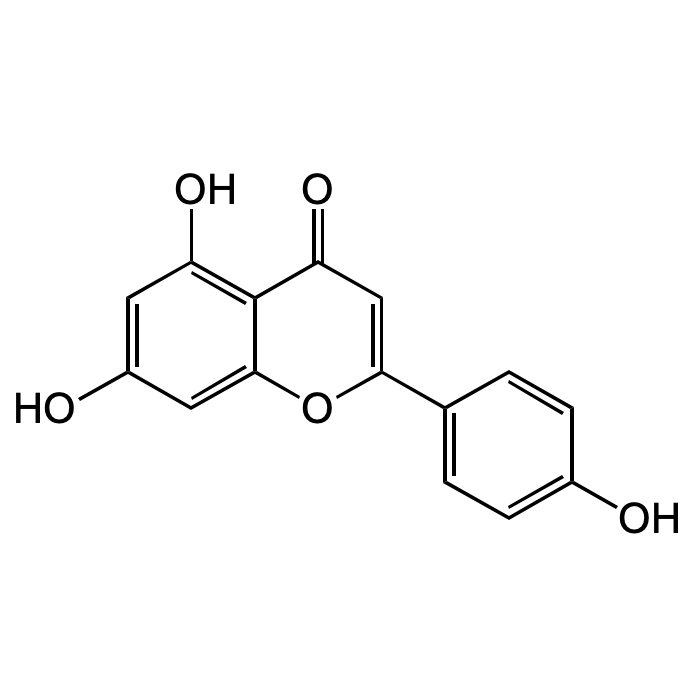
Apigenin
| Product Details | |
|---|---|
| Synonyms | Chamomile; Flavone; NSC 83244; Versulin; 4',5,7-Trihydroxyflavone; 5,7-Dihydroxy-2-(4-hydroxyphenyl)-4-benzopyrone |
| Product Type | Chemical |
| Properties | |
| Formula | C15H10O5 |
| MW | 270.24 |
| CAS | 520-36-5 |
| RTECS | LK9276000 |
| Source/Host Chemicals | Isolated from plant source. |
| Purity Chemicals | ≥95% (HPLC) |
| Appearance | Yellow to brown powder. |
| Solubility | Soluble in DMSO (10mg/ml) or DMF (20mg/ml). |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | KZNIFHPLKGYRTM-UHFFFAOYSA-N |
| Smiles | OC1=C2C(OC(C3=CC=C(O)C=C3)=CC2=O)=CC(O)=C1 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Apigenin is an antioxidant plant flavonoid with anti-inflammatory, angiogenic, neuroprotective and anticancer properties. It may induce apoptosis and inhibit proliferation of tumor cells by arresting the cell cycle at the G2/M phase. Inhibits tumor angiogenesis through decreasing HIF-1alpha and VEGF expression. Also known to be a MAP kinase inhibitor and a selectively inhibitor of casein kinase 2 (CK2). It is a potent inhibitor of the synthesis of the inflammatory mediators nitric oxide and prostaglandin E2, reducing inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) expression. Shown to selectively inhibits chymotrypsin-like and trypsin-like proteasome catalytic activity.
(1) Y.C. Liang, et al.; Carcinogenesis 20, 1945 (1999) | (2) D.H. Song, et al.; J. Biol. Chem. 275, 23790 (2000) | (3) J. Shen, et al.; J. Immunol. 167, 4919 (2001) | (4) G.M. Raso, et al.; Life Sci. 68, 921 (2001) | (5) M. Yamada, et al.; PNAS 102, 7736 (2005) | (6) M.B. Ujiki, et al.; Mol. Cancer 5, 76 (2006) | (7) J. Fang, et al.; Carcinogenesis 28, 858 (2006) | (8) Y.-X. Wu & X. Fang; Planta Med. 76, 128 (2010) | (9) X. Yan, et al.; Cell Biosci. 7, 50 (2017) (Review) | (10) S.F. Nabavi, et al.; Pharmacol. Res. 128, 359 (2018) (Review) | (11) J. Madunic, et al.; Cancer Lett. 413, 11 (2018) (Review)





