Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
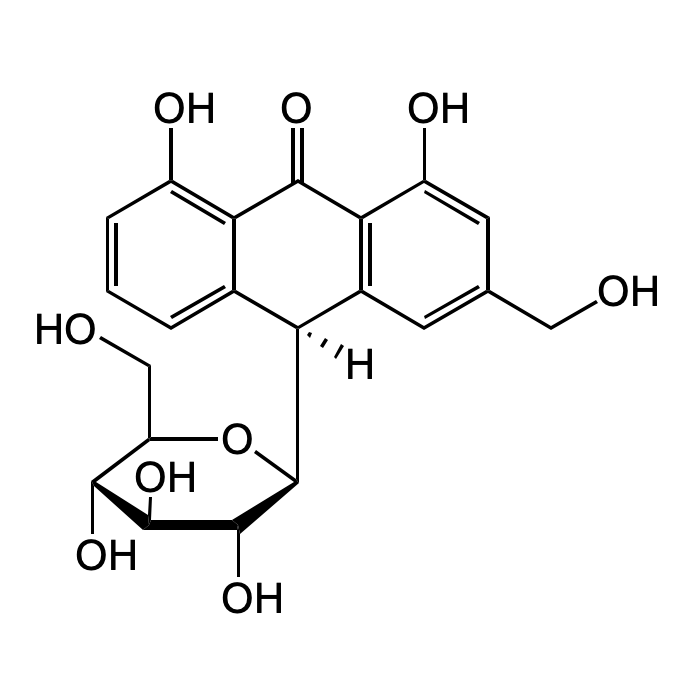
Aloin
| Product Details | |
|---|---|
| Synonyms | 1,8-Dihydroxy-10-(β-D-glucopyranosyl)-3-(hydroxymethyl)-9(10H)-anthracenone; Aloin A; Barbaloin A; NSC 407305 |
| Product Type | Chemical |
| Properties | |
| Formula | C21H22O9 |
| MW | 418.39 |
| CAS | 1415-73-2 |
| RTECS | LZ6520000 |
| Source/Host Chemicals | Isolated from plant source. |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | Yellow Powder. |
| Solubility | Soluble in DMSO or methanol (both 50mg/ml). |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | AFHJQYHRLPMKHU-OSYMLPPYSA-N |
| Smiles | OC1=C2C([C@@]([C@H]3[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O3)([H])C(C=C(CO)C=C4O)=C4C2=O)=CC=C1 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +20°C |
| Long Term Storage | +4°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at +4°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Aloin is a naturally occurring substance found in various Aloe plant species. Aloin was originally used as a laxative in the treatment of constipation. Aloin is a chemopreventive and antineoplastic agent. It is a cytotoxic against cancer cells. As a anticancer agent it inhibits Topoisomerase II alpha, induces apoptosis, targets High Mobility Group Box 1 (HMGB1) and inhibits angiogenesis. It has been shown to be a potent skin depigmenting agent, enhancing melanogenesis. It has been described as a tyroniase inhibitor. It regulates transglutaminase activity. In addition Aloin is an anti-inflammatory agent, inhibiting the NF-κB pathway, antitrypanosomal, iron chelating and microbiota modulating compound.
(1) C. Tan, et al.; Chin. Med. J. 115, 1859 (2002) | (2) A.Y. Esmat, et al.; Cancer Biol. Ther. 5, 97 (2006) | (3) A. Niciforovic, et al.; Cancer Biol. Ther. 6, 1200 (2007) | (4) E.J. Buenz; Toxicol. In Vitro 22, 422 (2008) | (5) A.H.A. Farooqi, et al.; J. Med. Aro. Plant. Sci. 31, 159 (2009) | (6) M.Y. Park, et al.; Biosci. Biotechnol. Biochem. 73, 828 (2009) | (7) S. Ravi, et al.; J. Exp. Sci. 2, 10 (2011) | (8) S. Hazrati, et al.; J. Med. Plants. Res. 6, 1834 (2012) | (9) S.A. Ali, et al.; Planta Med. 78, 767 (2012) | (10) Q. Pan, et al.; Cancer Cell Int. 13, 69 (2013) | (11) Y. Tewabe, et al.; BMC Vet. Res. 10, 61 (2014) | (12) A.Y. Esmat, et al.; Pharm. Biol. 53, 138 (2015) | (13) Y. Pengjam, et al.; Phytomedicine 23, 417 (2016) | (14) M.D. Boudreau, et al.; Toxicol. Sci. 158, 302 (2017) | (15) X. Luo, et al.; Molecules 23, E517 (2018) | (16) H. Tao, et al.; Drug Des. Devel. Ther. 13, 1221 (2019)





