Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
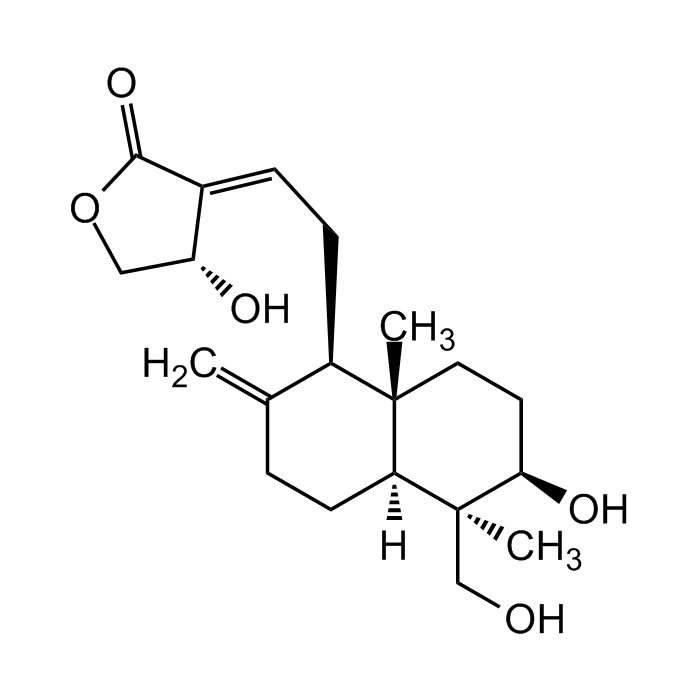
Andrographolide
| Product Details | |
|---|---|
| Synonyms | Andrographis |
| Product Type | Chemical |
| Properties | |
| Formula |
C20H30O5 |
| MW | 350.455 |
| CAS | 5508-58-7 |
| RTECS | LU3490750 |
| Source/Host Chemicals | Plant |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | White to light yellow powder. |
| Solubility | Soluble in chloroform, DMF (15mg/ml) or DMSO (2mg/ml). |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | BOJKULTULYSRAS-OTESTREVSA-N |
| Smiles | C=C1CC[C@@]([C@@](CO)(C)[C@H](O)CC2)([H])[C@]2(C)[C@@H]1C/C=C3C(OC[C@H]/3O)=O |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | -20°C |
| Long Term Storage | -20°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Andrographolide, a bioactive diterpene lactone, is the main constituent of Andrographis paniculata, a plant used in traditional medicines. It is known to have different biological properties such as anti-inflammatory, immunosuppressive, antidiabetic, antihyperglycemic, antibacterial, antioxidant, antinoniceptive, antimalarial, anticancer and hepatoprotective activity. It also shows potent anti-viral effect against dengue virus. It blocks T-cell proliferation and the proliferation of several cancer cell lines in vitro. It acts as an irreversible antagonist of NF-κ and AP-1 (IC50 ≤ 15 µM) activation, and prevents in vitro and in vivo T cell activation.
(1) P.K. Visen, et al.; J. Ethnopharmacol. 40, 131 (1993) | (2) W.F. Chiou, et al.; Br. J. Pharmacol. 129, 1553 (2000) | (3) B.C. Yu, et al.; Planta Med. 69, 1075 (2003) | (4) Y.F. Xia, et al.; J. Immunol. 173, 4207 (2004) | (5) J.H. Chen, et al.; Biochem. Pharmacol. 67, 1337 (2004) | (6) R.A. Burgos, et al.; Planta Med. 71, 429 (2005) | (7) M.I. Iruretagoyena, et al.; J. Pharmacol. Exp. Ther. 312, 366 (2005) | (8) C. Liu, et al.; Genes Cancer 2, 151 (2011) | (9) J.C. Lim, et al.; Clin. Exp. Pharmacol. Physiol. 39, 300 (2012) (Review) | (10) S.K. Mishra, et al.; Front. Biosci. 7, 255 (2015) (Review) | (11) V. Kishore, et al.; Curr. Top. Med. Chem. 17, 845 (2017) (Review) | (12) S. Gupta, et al.; Arch. Virol. 162, 611 (2017) (Review) | (13) W.S.D. Tan, et al.; Biochem. Pharmacol. 139, 71 (2017) (Review) | (14) C.H. Yang, et al.; Int. J. Mol. Sci. 18, E1638 (2017) (Review) | (15) M.T. Islam; Front. Pharmacol. 8, 571 (2017) (Review) | (16) J. Lu, et al.; Biomed. Pharmacother. 117, 109078 (2019) (Review) | (17) L. Zhang, et al.; Pharmacology (Epub ahead of print) (2019) (Review) | (18) E. Mussard, et al.; Antioxidants 8, E571 (2019) (Review)





