Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
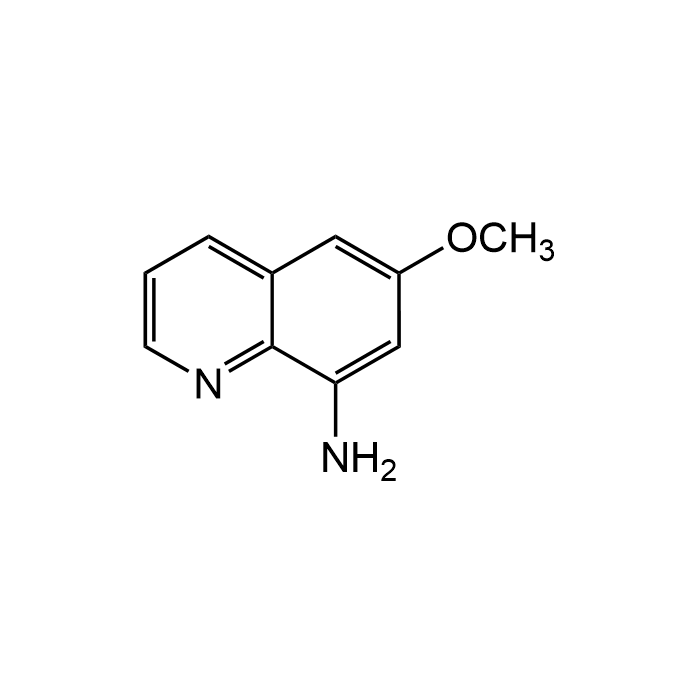
8-Amino-6-methoxyquinoline
As low as
32
CHF
CHF 32.00
In stock
Only %1 left
CDX-A0655-G0011 gCHF 32.00
CDX-A0655-G0055 gCHF 122.00
| Product Details | |
|---|---|
| Synonyms | 6-Methoxy-8-aminoquinoline; Amichin; WR15081; NSC 119507; NSC 119508; NSC 13573 |
| Product Type | Chemical |
| Properties | |
| Formula | C10H10N2O |
| MW | 174.2 |
| CAS | 90-52-8 |
| RTECS | VA9635000 |
| Source/Host Chemicals | Synthetic |
| Purity Chemicals | ≥95% (NMR) |
| Appearance | Dark green to dark brown solid. |
| Solubility | Soluble in chloroform or DMSO. |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | YGGTVPCTAKYCSQ-UHFFFAOYSA-N |
| Smiles | NC=1C2=C(C=C(OC)C1)C=CC=N2 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | +4°C |
| Handling Advice |
Keep under inert gas. Protect from light and oxygen. |
| Use/Stability | Stable for at least 2 years after receipt when stored at +4°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
8-Amino-6-methoxyquinoline is a versatile intermediate or buliding block in medicinal chemistry and analytical research. 8-Amino-6-methoxyquinoline serves as a key intermediate in the synthesis of various pharmaceuticals, particularly antimicrobial, antibacterial, antifungal, antimalarial or neuroprotective substances. It is employed in the development of fluorescent probes for biological imaging, allowing researchers to visualize cellular processes in real-time. It is used as a reagent in various analytical techniques, including chromatography and spectroscopy, to detect and quantify other chemical substances, enhancing the accuracy of analytical results.
Product References
(1) T.K. Li & L.J. Magnes; Biochem. Pharmacol. 21, 17 (1972) | (2) F.I. Carroll, et al.; J. Med. Chem. 22, 694 (1979) | (3) P.J. Zavala, et al.; Talanta 32, 285 (1985) | (4) P. Hochegger, et al.; Molecules 26, 5530 (2021)





