Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
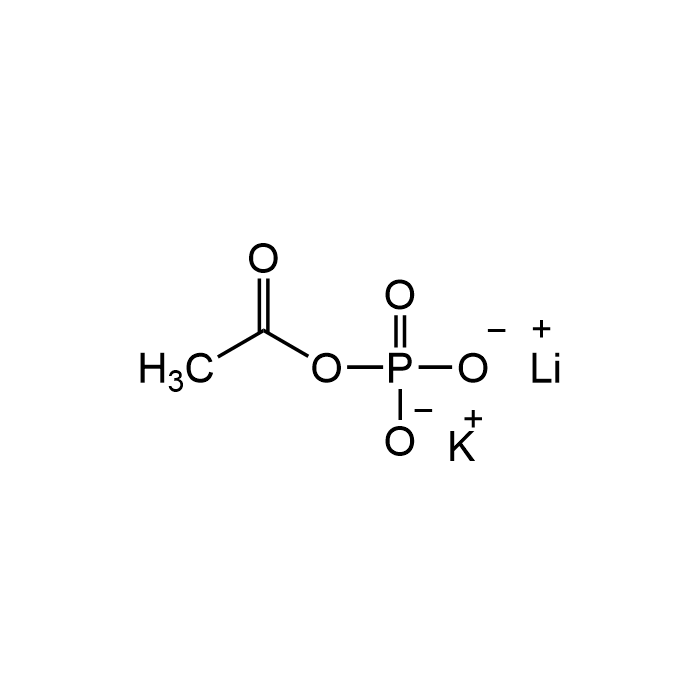
Acetyl phosphate lithium potassium
As low as
116
CHF
CHF 116.00
In stock
Only %1 left
CDX-A0781-M500500 mgCHF 116.00
CDX-A0781-G0011 gCHF 187.00
| Product Details | |
|---|---|
| Synonyms | Acetyl phosphate lithium potassium salt |
| Product Type | Chemical |
| Properties | |
| Formula | C2H3KLiO5P |
| MW | 184.06 |
| CAS | 94249-01-1 |
| Source/Host Chemicals | Synthetic |
| Appearance | White to off-white powder. |
| Solubility | Soluble in water (25mg/ml). |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | RLQMPLKXFIXRCV-UHFFFAOYSA-L |
| Smiles | [Li+].[K+].CC(=O)OP([O-])([O-])=O |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice |
Keep under inert gas. Protect from light and oxygen. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
Lithium potassium acetyl phosphate is an organometallic compound that is used as a phosphoryl group donor. It is commonly used in protein phosphorylation reactions. Lithium potassium acetyl phosphate is suitable as a source of acetyl phosphate. It is suitable for reducing inorganic monophosphate by-product formation by replacing inorganic oligophosphates in phosphatase-catalyzed transphosphorylation. It is a energy-rich compound for the regeneration of adenosine-5'-triphosphate, ATP, and other triphosphates. Lithium potassium acetyl phosphate is also used in research focused on energy storage. This compound is studied for its potential applications in the development of battery materials, particularly those involving lithium-ion technology. Researchers explore Lithium potassium acetyl phosphate for its electrochemical properties, such as ion conductivity and stability, which are for improving battery performance and longevity.
Product References
(1) W.R. Moore & A. Meister; Anal. Biochem. 161, 487 (1987) | (3) D.C. Crans, et al.; Meth. Enzymol. 136, 263 (1987) | (4) D. Janasek, et al.; Anal. Bioanal. Chem. 374, 1267 (2002) | (5) S. Mukhopadhyay, et al.; Bioorg. Chem. 36, 65 (2008) | (6) Y.H. Liu, et al.; J. Mol. Biol. 391, 149 (2009) | (7) Q. Chen, et al.; Bio. Protoc. 3, e970 (2013) | (8) B. Yan, et al.; World J. Microbiol. Biotechnol. 30, 1123 (2014) | (9) E. Kinoshita-Kikuta, et al.; PloS one 10, e0132598 (2015) | (10) G. Tasnadi, et al.; Adv. Synth. Catal. 360, 2394 (2018) | (11) A. Karami, et al: Front. Aging Neurosci. 13, 704583 (2021) | (12) E. Camprubi, et al.; Astrobiol. 22, 981 (2022)





