Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
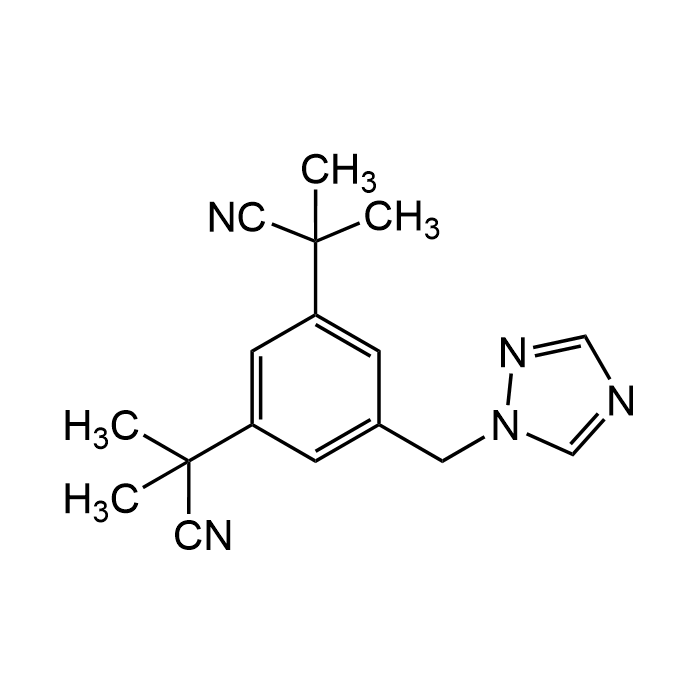
Anastrozole
| Product Details | |
|---|---|
| Synonyms | Anastrol; ICI-D 1033; ZD 1033; α,α,α',α'-Tetramethyl-5-(1H-1,2,4-triazol-1-ylmethyl)-1,3-benzenediacetonitrile |
| Product Type | Chemical |
| Properties | |
| Formula | C17H19N5 |
| MW | 293.37 |
| CAS | 120511-73-1 |
| RTECS | CZ1465000 |
| Source/Host Chemicals | Synthetic |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | White crystalline powder. |
| Solubility | Soluble in DMF (10 mg/ml), DMSO (10 mg/ml), ethanol (20 mg/ml), methanol or chloroform. |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | YBBLVLTVTVSKRW-UHFFFAOYSA-N |
| Smiles | CC(C)(C#N)C1=CC(CN2C=NC=N2)=CC(C(C)(C)C#N)=C1 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +20°C |
| Long Term Storage | +4°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at +4°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Anastrozole is a highly potent, non-steroidal aromatase inhibitor that selectively inhibits the aromatase enzyme (CYP19A1) responsible for converting androgens to estrogens. With an IC50 in the low nanomolar range (typically ~15 nM), it effectively suppresses estrogen synthesis, making it a key compound in hormone-related cancer research. It is selective for aromatase/CYP19A1 over the cytochrome P450 (CYP) isoforms CYP1A2, CYP2A6, CYP2C9, CYP2D6, and CYP3A. Anastrozole is widely used in studies of estrogen receptor-positive (ER+) breast cancer, endocrine resistance and hormone regulation. It is a standard reference compound in preclinical drug screening and mechanistic studies targeting estrogen biosynthesis. Compound can be used as analytical reference material in relation to doping analytics.





