Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
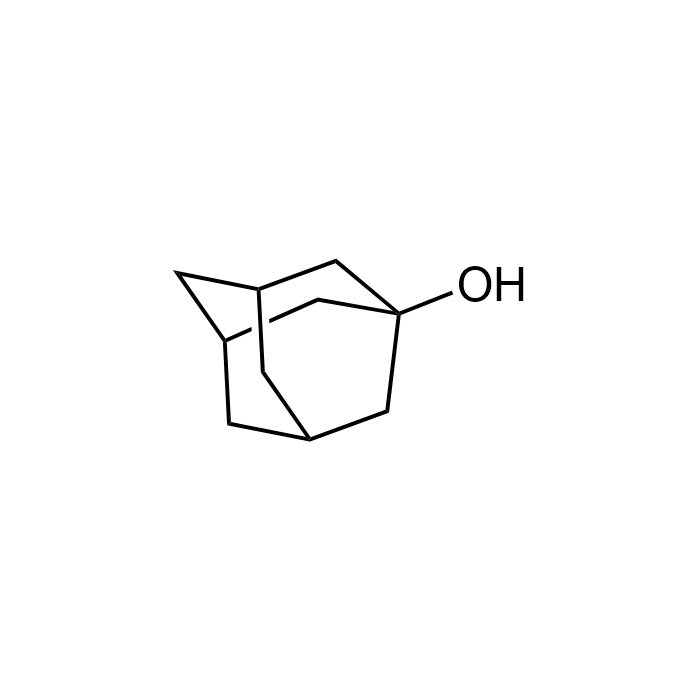
1-Adamantanol
As low as
77
CHF
CHF 77.00
In stock
Only %1 left
CDX-A0811-G02525 gCHF 77.00
| Product Details | |
|---|---|
| Synonyms | 1-Hydroxyadamantan; 1-Adamantyl alcohol; Adamantan-1-ol; NSC 108837 |
| Product Type | Chemical |
| Properties | |
| Formula | C10H16O |
| MW | 152.23 |
| CAS | 768-95-6 |
| RTECS | AU4980000 |
| Source/Host Chemicals | Synthetic |
| Purity Chemicals | ≥99% (GC) |
| Appearance | White to off-white powder or crystals. |
| Solubility | Soluble in chloroform, methanol or ethanol. Insoluble in water. |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | VLLNJDMHDJRNFK-URIMCQPQSA-N |
| Smiles | OC12C[C@H](C3)C[C@@H](C2)C[C@H]3C1 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
1-Adamantanol is a compound that functions as a versatile building block in organic synthesis. It serves as a precursor for the preparation of various derivatives, including polymers, and specialty chemicals. Its mechanism of action involves participating in a range of chemical reactions, such as esterification, etherification and oxidation, to yield structurally diverse products. 1-Adamantanol's molecular structure allows it to undergo selective functionalization, enabling the creation of new compounds with tailored properties. Its role in the synthesis of complex molecules may be useful for exploring the structure-activity relationships of organic compounds. At the molecular level, 1-Adamantanol's functional groups facilitate its reactivity, leading to the formation of novel chemical entities with potential applications in various fields of study.
Product References
(1) K. Mitsukura, et al.; J. Biosci. Bioeng. 109, 550 (2010) | (2) A.A. Awasthi, et al.; Chemphyschem. 22, 975 (2021) | (3) J.W. Wang, et al.; Molecules 26, 2412 (2021) | (4) K.L. Mears, et al.; Angew. Chem. Int. Ed. Engl. 61, e202201318 (2022) | (5) F.T. Meng, et al.; Nat. Commun. 13, 7393 (2022) | (6) P. Jesionek, et al.; Spectrochim. Acta A Mol. Biomol. Spectrosc. 299, 122794 (2023)





