Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
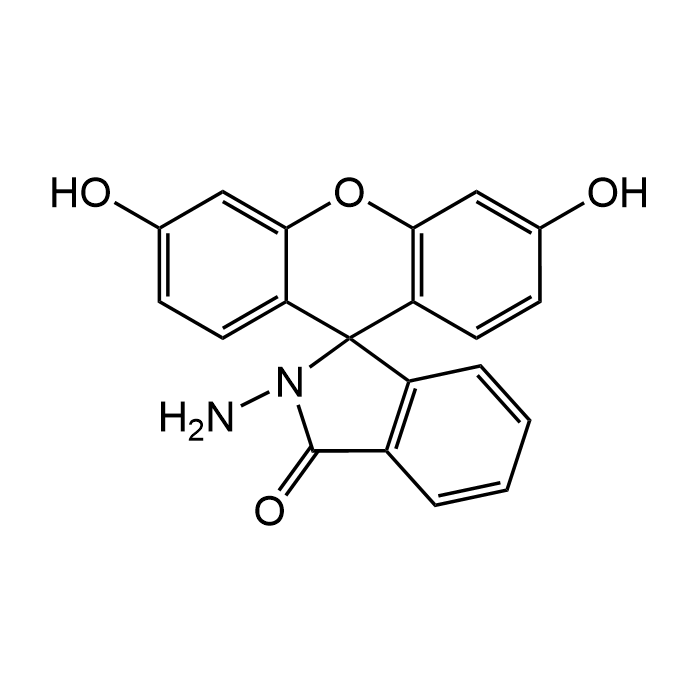
N-Aminofluorescein
As low as
161
CHF
CHF 161.00
In stock
Only %1 left
CDX-A0936-M05050 mgCHF 161.00
CDX-A0936-M100100 mgCHF 258.00
| Product Details | |
|---|---|
| Synonyms | 2-Amino-3',6'-dihydroxyspiro[isoindole-3,9'-xanthene]-1-one; 2-Amino-3',6'-dihydroxyspiro[isoindoline-1,9'-xanthen]-3-one; NSC 374134 |
| Product Type | Chemical |
| Properties | |
| Formula | C20H14N2O4 |
| MW | 346.34 |
| CAS | 98907-26-7 |
| Source/Host Chemicals | Synthetic |
| Purity Chemicals | ≥98% |
| Appearance | Off white to slight yellow powder. |
| Solubility | Soluble in DMSO (50mg/ml). |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | CHYVTSCIBXXQJT-UHFFFAOYSA-N |
| Smiles | O=C(C1=C2C=CC=C1)N(N)C32C4=CC=C(O)C=C4OC5=C3C=CC(O)=C5 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +20°C |
| Long Term Storage | +4°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at +4°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
N-Aminofluorescein is a fluorescein hydrazide with spiro ring structure and described as a highly selective and sensitive fluorescence probe for Cu2+. It absorbs maximally around 490-495 nm and emits bright green fluorescence near 515-520 nm, with a high quantum yield. It can be used for direct detection of Cu2+ in biological systems. Spectral Data: λex/em = 495/516nm. The presence of the exocyclic amino group in the structure, allows for selective chemical reactivity, making it suitable for use as a building block in the design of redox-sensitive probes, labeling reagents, and diagnostic sensors.
Product References
(1) M. Adamczyk & J. Grote; Tetrahedr. Lett. 41, 807 (2000) | (2) X.-F. Yang, et al.; Microchim. Acta 149, 123 (2005) | (3) X. Chen & H. Ma; Anal. Chim. Acta 575, 217 (2006) | (4) X.-F. Yang, et al.; Anal. Chim. Acta 584, 95 (2007) | (5) T. Li, et al.; Dyes Pigm. 88, 103 (2011) | (6) C. Shen, et al.; Chem. Commun. 51, 6312 (2015) | (7) C. Cetintas, et al.; Sens. Actuat. B: Chem. 234, 109 (2016) | (8) Y.L. Chen, et al.; J. Luminescence 275, 120747 (2024) | (9) H.T. Zhi, et al.; Bioorg. Chem. 147, 107421 (2024)





