Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
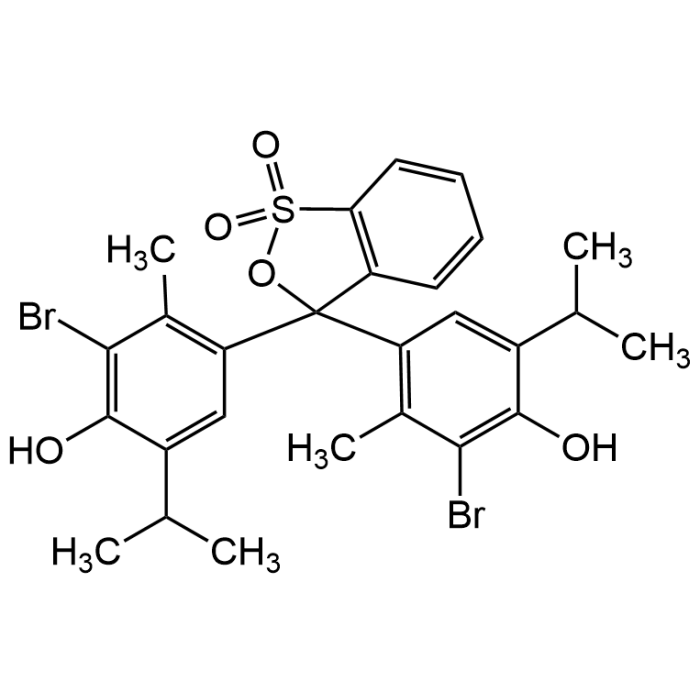
Bromothymol Blue
As low as
77
CHF
CHF 77.00
In stock
Only %1 left
CDX-B0049-G02525 gCHF 77.00
CDX-B0049-G100100 gCHF 232.00
| Product Details | |
|---|---|
| Synonyms | 3,3′-Dibromothymolsulfophthalein; Bromothymolsulfonephthalein; NSC 7819 |
| Product Type | Chemical |
| Properties | |
| Formula | C27H28Br2O5S |
| MW | 624.38 |
| CAS | 76-59-5 |
| RTECS | SJ7450000 |
| Source/Host Chemicals | Synthetic |
| Purity Chemicals | ≥95% (Dye content) |
| Appearance | Violet powder. |
| Solubility | Soluble in DMSO. Sparingly soluble in water. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | NUHCTOLBWMJMLX-UHFFFAOYSA-N |
| Smiles | O=S1(OC(C2=C(C)C(Br)=C(O)C(C(C)C)=C2)(C3=C(C)C(Br)=C(O)C(C(C)C)=C3)C4=C1C=CC=C4)=O |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +20°C |
| Long Term Storage | +20°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at RT. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
Bromothymol Blue (BTB) is a sulfonephthalein-based pH indicator dye with a sharp and reversible color transition near neutrality: yellow in acidic conditions, blue in alkaline media, with a clear transition interval around pH 6.0-7.6. Bromothymol Blue is widely applied in analytical chemistry and education as a reliable acid-base indicator for titrations and demonstrations of pH changes. In microbiology and biotechnology it is incorporated into culture media to reveal microbial metabolism, acid formation, or carbon dioxide release, while in environmental monitoring it serves as a practical tool for assessing water quality and maintaining stable pH in aquaculture systems. The compound also finds use in physiological and medical research where it assists in the study of respiration, photosynthesis, and other pH-sensitive biochemical processes. Beyond the laboratory, Bromothymol Blue is a common component of indicator solutions and pH testing kits.
Product References
(1) J.B. Jackson & A.R. Crofts; Eur. J. Biochem. 10, 226 (1969) | (2) Z. Aleksandrowicz & J. Swierczynski; FEBS Lett, 20, 364 (1972) | (3) J.B. Puschett, et al.; Talanta 38, 335 (1991) | (4) R.W. Sabnis; Handbook of Acid-Base Indicators (2007) | (6) R.W. Sabnis; Handbook of biological dyes and stains (2010)





