Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
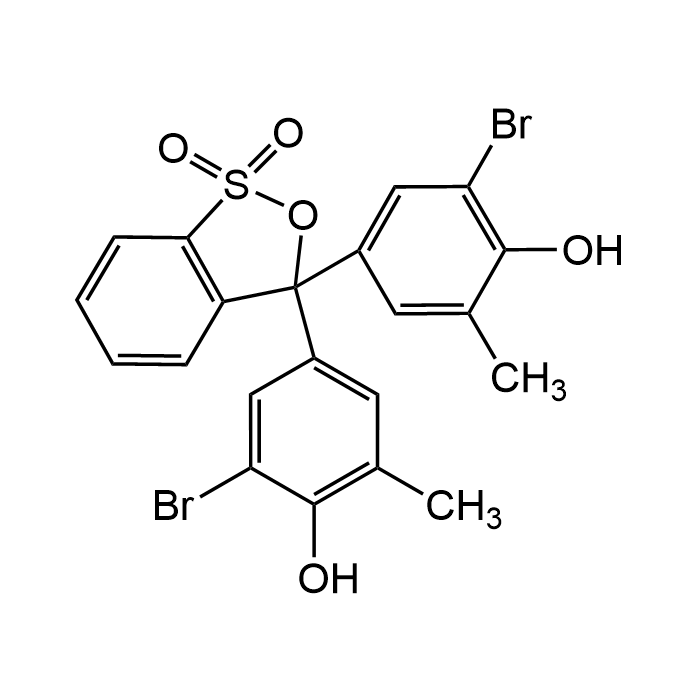
Bromocresol Purple
As low as
45
CHF
CHF 45.00
In stock
Only %1 left
CDX-B0055-G01010 gCHF 45.00
CDX-B0055-G05050 gCHF 180.00
| Product Details | |
|---|---|
| Synonyms | NSC 374134; BCP; 5,5'-Dibromo-o-cresolsulfonphthalein; |
| Product Type | Chemical |
| Properties | |
| Formula | C21H16Br2O5S |
| MW | 540.22 |
| CAS | 115-40-2 |
| Source/Host Chemicals | Synthetic |
| Purity Chemicals | ≥94% (Assay) |
| Appearance | Solid. |
| Solubility | Soluble in ethanol (20 mg/ml), methanol or DMSO. Insoluble in water. |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | ABIUHPWEYMSGSR-UHFFFAOYSA-N |
| Smiles | BrC1=C(O)C(C)=CC(C(OS2(=O)=O)(C3=CC(C)=C(O)C(Br)=C3)C4=C2C=CC=C4)=C1 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +20°C |
| Long Term Storage | +4°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at +4°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
Bromocresol Purple (BCP) is a pH-sensitive dye widely used as a colorimetric pH indicator and analytical reagent. It exhibits a distinct color change from yellow at pH <5.2 to purple at pH >6.8, making it valuable in titrations, buffer preparation and biochemical assays. In clinical and laboratory settings, it is commonly used in protein and albumin assays due to its ability to form colored complexes under acidic conditions. In yeast it helps in dead cell count for cells with plasma membrane damage. Cells with proper cell membrane are not stained, while damaged cells are seen as blue-grey ghosts.
Product References
(1) R.M. Tel, et al.; J. Clin. Chem. Clin. Biochem. 17, 627 (1979) | (2) P.G. Hill; Ann. Clin. Biochem. 22, 565 (1985) | (3) H. Kurzweilova & K. Sigler; Yeast 9, 1207 (1993) | (4) B.T. Doumas & T. Peters; Clin. Chim. Acta 258, 3 (1997) | (5) S. Ito & D. Yamamoto; Clin. Chim. Acta 411, 294 (2010) | (6) A.L. Silva, et al.; Bio. Protoc. 8, e2796 (2018) | (7) S. Yoshihiro, et al.; J. Clin. Biochem. Nutr. 67, 257 (2020) | (8) H. Khanjanzadeh & B.D. Park; Carbohydr. Polym. 273, 118550 (2021)





