Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
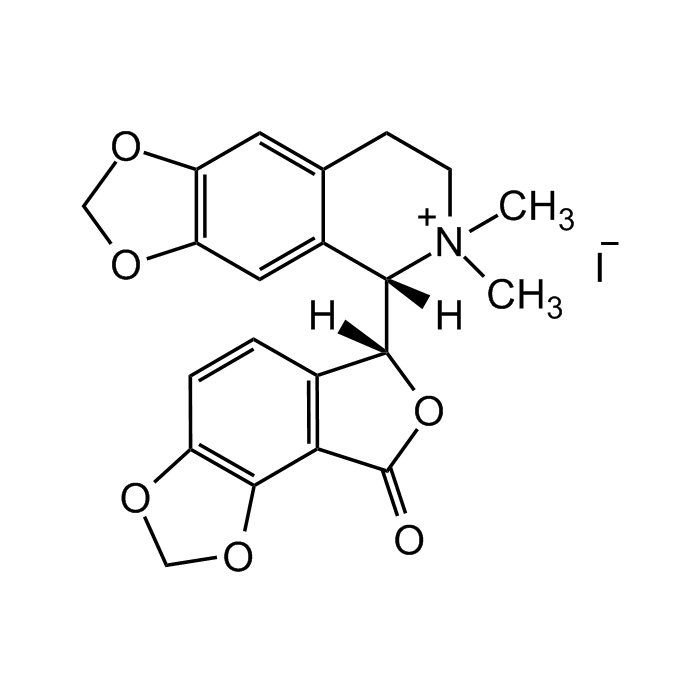
(-)-Bicuculline methiodide
| Product Details | |
|---|---|
| Synonyms | N-Methylbicuculline iodide; 55950-07-7 |
| Product Type | Chemical |
| Properties | |
| Formula |
C21H20INO6 |
| MW | 509.29 |
| CAS | 40709-69-1 |
| Purity Chemicals | ≥95% (NMR) |
| Appearance | White to off-white powder. |
| Solubility | Soluble in water (10 mg/ml) or DMSO (30mg/ml). |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | HKJKCPKPSSVUHY-GRTNUQQKSA-M |
| Smiles | C[N+]1(C)[C@@]([C@@]2([H])C(C=CC3=C4OCO3)=C4C(O2)=O)([H])C5=CC6=C(OCO6)C=C5CC1.[I-] |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +20°C |
| Long Term Storage | +4°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at +4°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
(-)-Bicuculline methiodide is the N-methylated water-soluble derivative of the widely-employed ionotropic GABAA receptor antagonist (+)-Bicuculline. This prototypic, competitive GABAA receptor antagonist displaces GABA from the agonist binding site to prevent receptor activation. It also acts as a negative allosteric inhibitor of channel opening to inhibit GABAA receptor activation by anaesthetic agents. Additionally it shows activity at SK calcium-activated potassium channels, nicotinic acetylcholine receptors and acetylcholinesterase. This compound reversibly and competitively blocks GABAA receptor mediated currents and is widely used to isolate glutamate receptor mediated EPSCs (excitatory postsynaptic potentials).
(1) R.W. Olsen, et al.; Brain Res. 102, 283 (1976) | (2) V. Seutin & S.W. Johnson; TiPS 20, 268 (1999) | (3) O. Chan, et al.; Diabetes 55, 1080 (2006) | (4) S. Kurt, et al.; Hear Res. 212, 224 (2006) | (5) G.A. Johnston; Br. J. Pharmacol. 169, 328 (2013) | (6) R.W. Olsen; Neuropharmacol. 136, 10 (2018)





