Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
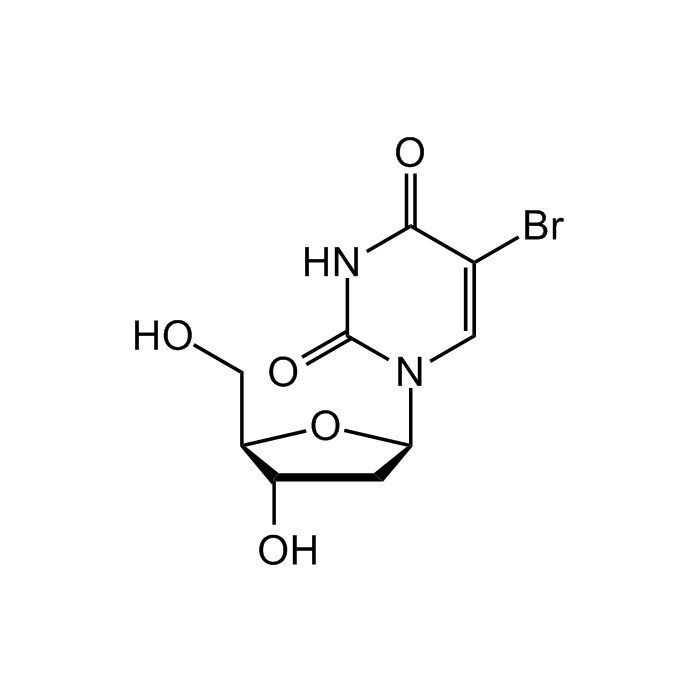
5-Bromo-2'-deoxyuridine
| Product Details | |
|---|---|
| Synonyms | 5-BrdU; 5-Bromo-1-(2-deoxy-β-D-ribofuranosyl)uracil; 5-Bromouracil deoxyriboside; BUdR; Bromodeoxyuridine; Broxuridine; NSC 38297 |
| Product Type | Chemical |
| Properties | |
| Formula |
C9H11BrN2O5 |
| MW | 307.1 |
| CAS | 59-14-3 |
| RTECS | YU7350000 |
| Purity Chemicals | ≥99% (HPLC) |
| Appearance | White powder. |
| Solubility | Soluble in DMSO (30mg/ml), DMF (30mg/ml) or ethanol (25mg/ml) or water (10mg/ml). |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | WOVKYSAHUYNSMH-RRKCRQDMSA-N |
| Smiles | O[C@H]1C[C@H](N2C=C(Br)C(NC2=O)=O)O[C@@H]1CO |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
The synthetic nucleoside 5-Bromo-2'-deoxyuridine (BrdU) is a thymidine analog used to label DNA and commonly used in the detection of proliferating cells in living tissues. It can be incorporated into newly synthesized DNA in place of thymidine during the S phase of the cell cycle. Cells that were actively proliferating can then be detected by denaturing the DNA and allowing specific antibodies with fluorescent tags to target the BrdU incorporation for detection via flow cytometry or fluorescence microscopy. Binding of the antibody requires denaturation of the DNA, usually by exposing the cells to acid or heat. BrdU is also used as a mutagen in genetic research. BrdU stimulates cellular differentiation and maturation in leukemia cell lines, while it inhibits differentiation of friend erythroleukemia cells. BrdU replaces Oct-4 in transcription factor-mediated reprogramming of somatic cells and can be used in small molecule cocktail to generate ciPSCs.
(1) N. Kee, et al.; J. Neurosci. Methods 115, 97 (2002) | (2) R.C. Leif, et al.; Cytometry A 58, 45 (2004) (Review) | (3) P. Taupin; Brain Res. Rev. 53, 198 (2007) (Review) | (4) D.W. Stacey & M. Hitomi; Cytometry A 73, 270 (2008) | (5) B.L. Cavanagh, et al.; Molecules 16, 7980 (2011) | (6) B. Lehner, et al.; Cell Tissue Res. 345, 313 (2011) | (7) T. Konishi, et al.; J. Radiat. Res. 52, 433 (2011) | (8) J.M. Barker, et al.; PLoS One 8, e63692 (2013) | (9) Y. Long, et al.; Cell Res. 25, 1171 (2015)





