Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
Bicalutamide
As low as
155
CHF
CHF 155.00
In stock
Only %1 left
CDX-B0305-G0011 gCHF 155.00
CDX-B0305-GG252.5 gCHF 309.00
| Product Details | |
|---|---|
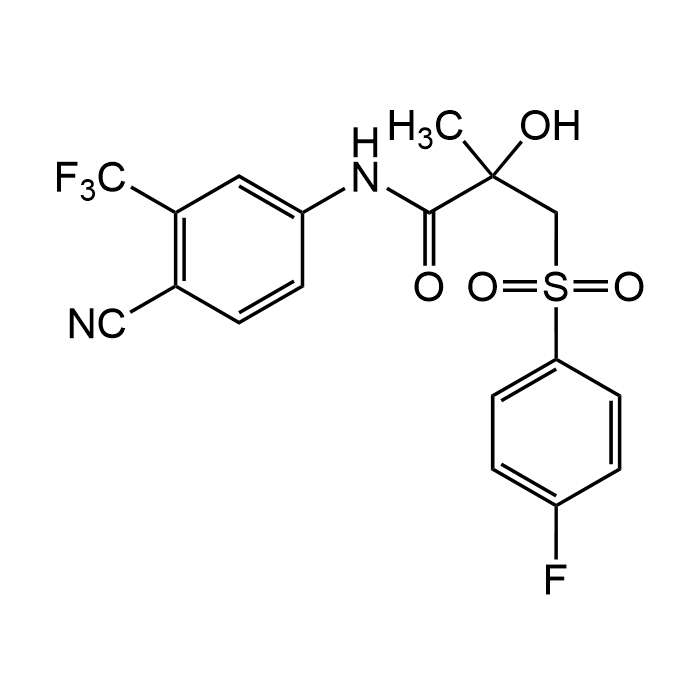
| Synonyms | ICI 176334; ZD 176334; Casodex; Cosudex; N-[4-Cyano-3-(trifluoromethyl)phenyl]-3-[(4-fluorophenyl)sulfonyl]-2-hydroxy-2-methylpropanamide |
| Product Type | Chemical |
| Properties | |
| Formula | C18H14F4N2O4S |
| MW | 430.37 |
| CAS | 90357-06-5 |
| RTECS | TX1413500 |
| Source/Host Chemicals | Synthetic |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | White to off-white powder. |
| Solubility | Soluble in DMF (20 mg/ml) or DMSO (10 mg/ml). Slightly soluble in ethanol (1 mg/ml). |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | LKJPYSCBVHEWIU-UHFFFAOYSA-N |
| Smiles | O=C(C(C)(O)CS(=O)(C1=CC=C(F)C=C1)=O)NC2=CC(C(F)(F)F)=C(C#N)C=C2 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
Bicalutamide is a potent, non-steroidal anti-androgen that functions as a selective androgen receptor (AR) antagonist, widely used in prostate cancer research and studies involving androgen signaling. It binds competitively to the androgen receptor with high affinity, blocking the action of endogenous androgens such as testosterone and dihydrotestosterone, effectively suppressing androgen-driven cellular processes. Bicalutamide is active in both in vitro and in vivo models and demonstrates good oral bioavailability and metabolic stability. Bicalutamide is frequently used to examine the role of androgen receptor inactivation in the proliferation of prostate cancer cells and has served as a molecular template for the design and structural optimization of more selective androgen receptor modulators for androgen therapy.
Product References
(1) S.N. Freeman, et al.; Br. J. Cancer 60, 664 (1989) | (2) D. Masiello, et al.; J. Biol. Chem. 277, 26321 (2002) | (3) D. Yin, et al.; Mol. Pharmacol. 63, 211 (2003) | (4) C.E. Bohl, et al.; PNAS 102, 6201 (2005) | (5) W. Gao, et al.; Pharmacol. Res. 23, 1641 (2006) | (6) C.N. Sternberg; Nat. Clin. Pract. Urol. 3, 408 (2006) | (7) C. Festuccia, et al.; Prostate 67, 1255 (2007) | (8) D.J. Osguthorpe & A.T. Hagler; Biochemistry 50, 4105 (2011) | (9) N.S. Nourbakhsh, et al.; Tissue Cell 91, 102530 (2024)





