Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
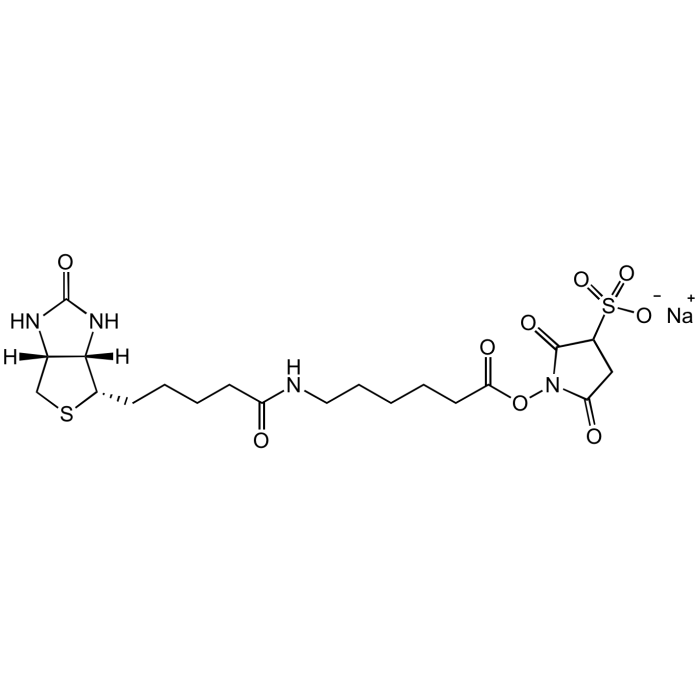
Sulfo-NHS-LC-Biotin . Na
| Product Details | |
|---|---|
| Synonyms | EZ-Link Sulfo-NHS-LC-Biotin; Sulfosuccinimidyl 6-(biotinamido)hexanoate; Biotinamidohexanoic acid 3-sulfo-N-hydroxysuccinimide ester sodium salt |
| Product Type | Chemical |
| Properties | |
| Formula |
C20H29N4NaO9S2 |
| MW | 556.59 |
| CAS | 191671-46-2 | 127062-22-0 |
| Source/Host Chemicals | Synthetic. |
| Purity Chemicals | ≥90% (NMR) |
| Appearance | White to off-white powder. |
| Solubility | Soluble in water (10mg/ml), DMSO or DMF. |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | JJGWLCLUQNFDIS-GTSONSFRSA-M |
| Smiles | O=C(NCCCCCC(ON1C(CC(S(=O)([O-])=O)C1=O)=O)=O)CCCC[C@@H]2SC[C@@]3([H])[C@]2([H])NC(N3)=O.[Na+] |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Sulfo-NHS-LC-Biotin is an amino-reactive biotinylation reagent with a long spacer arm. The 6-aminocaproic acid spacer arm provides distance between the biotin and the target ligand which helps alleviate steric hindrance, in addition it forms permanent amide bonds. The sodium sulfonate groups render this compound water-soluble and prevent it from crossing the lipid bi-layer of the cell membrane, making it a tool to label cell surface proteins. In addition, Sulfo-NHS-LC-Biotin is used to biotinylate antibodies, proteins, peptides, or amino acids by reacting with primary amines as found in the N-terminus and Lysine side chains. Optimal reaction conditions are at a pH of 7-9. Avoid amine-containing buffers, which may compete in the acylation reaction.
(1) G.T. Hermanson; Bioconjugate Techn. 2nd p506-545 (2008)





